Abstract
Background:
In current practice, the status of residual low-frequency acoustic hearing in hearing preservation cochlear implantation (CI) is unknown until activation two to three weeks postoperatively. The intraoperatively measured electrically evoked compound action potential (ECAP), a synchronous response from electrically stimulated auditory nerve fibers, is one of the first markers of auditory nerve function after cochlear implant surgery and such may provide information regarding the status of residual low-frequency acoustic hearing.
Purpose:
This study aimed to evaluate the relationship between intraoperative ECAP at the time of CI and presence of preoperative and postoperative low-frequency acoustic hearing.
Research Design:
A retrospective case review.
Study Sample:
Two hundred seventeen adult ears receiving CI (42 Advanced Bionics, 82 Cochlear, and 93 MED-EL implants).
Interventions:
Intraoperative ECAP and CI.
Data Collection and Analysis:
ECAP measurements were obtained intraoperatively, whereas residual hearing data were obtained from postoperative CI activation audiogram. A linear mixed model test revealed no interaction effects for the following variables: manufacturer, electrode location (basal, middle, and apical), preoperative low-frequency pure-tone average (LFPTA), and postoperative LFPTA. The postoperative residual low-frequency hearing status was defined as preservation of unaided air conduction thresholds ≤90 dB at 250 Hz. Electrode location and hearing preservation data were analyzed individually for both the ECAP threshold and ECAP maximum amplitude using multiple t-tests, without assuming a consistent standard deviation between the groups, and with alpha correction.
Results:
The maximum amplitude, in microvolts, was significantly higher throughout apical and middle regions of the cochlea in patients who had preserved low-frequency acoustic hearing as compared with those who did not have preserved hearing (p = 0.0001 and p = 0.0088, respectively). ECAP threshold, in microamperes, was significantly lower throughout the apical region of the cochlea in patients with preserved low-frequency acoustic hearing as compared with those without preserved hearing (p = 0.0099). Basal electrode maximum amplitudes and middle and basal electrode thresholds were not significantly correlated with postoperative low-frequency hearing.
Conclusions:
Apical and middle electrode maximum amplitudes and apical electrode thresholds detected through intraoperative ECAP measurements are significantly correlated with preservation of low-frequency acoustic hearing. This association may represent a potential immediate feedback mechanism for postoperative outcomes that can be applied to all CIs.
Keywords: cochlear implantation, electrically evoked compound action potential, hearing preservation, maximum amplitude, residual hearing, threshold
INTRODUCTION
In recent years, minimally traumatic surgical techniques, hearing preservation electrodes, and hybrid electric–acoustic processors have expanded the indications for cochlear implantation (CI). This trend is supported by growing evidence, indicating that combined electric and acoustic stimulation lends to improved speech recognition in complex listening environments, sound localization, music appreciation, and decreased listening effort (Turner et al, 2008; Buchner et al, 2009; Gifford et al, 2013; 2017). Hearing preservation after cochlear implant surgery is facilitated by electrode design and surgical technique, minimizing insertion trauma (Nadol et al, 1989; O’Connell et al, 2016; Wanna et al, 2018; Bruce and Todt, 2018). Despite these advances, the degree of hearing preservation varies significantly across individuals (Gantz et al, 2016; Helbig et al, 2016; Hunter et al, 2016; Moteki et al, 2017).
Postoperative acoustic hearing loss typically falls into two categories: immediate and delayed. Immediate-onset hearing loss that is detected at the first postoperative appointment is commonly attributed to surgical trauma or an acute inflammatory process (Eshraghi et al, 2005; Carlson et al, 2011; Seyyedi and Nadol, 2014). Presently, the status of acoustic hearing after CI is unknown before the activation audiogram typically completed three weeks postoperatively. Electrocochleography has been proposed for real-time feedback during electrode insertion, but it is not available with all electrode designs or precisely correlated with hearing preservation outcomes (Kim et al, 2017; O’Connell et al, 2017a,b). The causes of delayed-onset hearing loss have yet to be elucidated, but it has been hypothesized that a foreign body reaction to the electrode array may be involved (Seyyedi and Nadol, 2014).
The electrically evoked compound action potential (ECAP) measured intraoperatively is one of the first markers of auditory nerve function after cochlear implant surgery. The ECAP represents a synchronous response from electrically stimulated auditory nerve fibers, providing information regarding the status of the auditory nerve. ECAP measurements are used intraoperatively to confirm auditory nerve, device integrity, and electrode functionality and postoperatively by audiologists for speech processor programming. ECAP measurements have been correlated with both detection thresholds (T-levels) and maximum comfortable loudness (C-levels), with a greater correlation found with T-levels (Brown et al, 1996; 1998; 2000; Hughes et al, 2000; Franck and Norton, 2001; Abbas et al, 2017). The ECAP maximum amplitude has been linked to speech perception scores after CI (Kim et al, 2010; 2017; Schvartz-Leyzac and Pfingst, 2018). In addition, patients with low behavioral audiologic thresholds and larger ECAP amplitudes are more likely to have higher speech perception scores (DeVries et al, 2016).
Although postoperative ECAP measurements have been previously associated with speech perception scores, the clinical utility of intraoperative ECAP measurements have been limited to date. In patients with residual acoustic hearing in the apical cochlea, the ECAP response can theoretically originate from stimulation of the spiral ganglion cells in the modiolus and from direct intracochlear electrical stimulation of inner hair cells in the scala media. Auditory stimulation resulting from electrical stimulation of spiral ganglion cells is often referred to as electroneural hearing, whereas auditory stimulation resulting from electrical stimulation of inner hair cells is referred to as electrophonic hearing. Several studies have documented electrophonic auditory activity with intact intracochlear structures such that the electrical stimulation resulted in basilar membrane mechanical activation with peak activity corresponding to the frequency of the stimulus (Kirk and Yates, 1994; Nuttall and Ren, 1995; Xue et al, 1995; Sato et al, 2016). Thus, in this patient population with preserved low-frequency acoustic hearing and, hence, preserved apical inner hair cells, ECAPs are likely resulting from both electroneural and electrophonic stimulation.
Although routinely obtained for cochlear implant patients at many centers, the role of intraoperative ECAP measurements for traditional and hearing preservation CI has not been previously reported. This study investigates the association between intraoperative ECAP measurements after electrode insertion and postoperative audiologic outcomes in patients with and without residual low-frequency acoustic hearing. Our primary hypothesis was that patients with more robust intraoperative ECAP responses (e.g., lower ECAP thresholds and higher ECAP amplitudes) would have better rates of postoperative acoustic hearing preservation.
MATERIALS AND METHODS
Patient Selection and Clinical Information
Institutional review board approval was obtained before initiation of the study. Adult patients were included if they (a) underwent CI via mastoidectomy and facial recess approach, (b) had intraoperative ECAP measurements, and (c) had postoperative activation audiogram, confirming the presence or absence of residual low-frequency unaided air conduction thresholds. Both hearing preservation candidates and traditional candidates were included. Patients were excluded if records were incomplete, with one exception: some patients only had available ECAP thresholds or amplitudes, and these patients were included for their respective analyses. Notably, all patients were implanted using a “soft surgical” technique to preserve cochlear structural preservation, irrespective of the preoperative hearing preservation status. A round window approach was always preferred and attempted, with an extended round window or a cochleostomy approach performed as an alternative if a round window approach was not feasible. Intraoperative ECAP measurements were recorded before the termination of general anesthesia using a clinical protocol that attempted to deliver similar levels of charge across all patients. ECAPs were recorded via standard clinical software for all implant manufacturers using biphasic pulses with a monopolar electrode configuration. Stimulation rates across manufacturers were 32, 80, and 80 pulses per second for Advanced Bionics (Valencia, CA), Cochlear (Englewood, CO), and MED-EL (Innsbruck, AT), respectively. Pulse durations were 32, 25, and 30 μsec per phase for Advanced Bionics, Cochlear, and MED-EL, respectively. ECAP parameters were not chosen a priori, but rather reflect data collected per clinical protocol, allowing this retrospective review. For Advanced Bionics, we collect intraoperative ECAP data on odd electrodes plus electrode 16 using stimulation levels 100 to 500 CU, in 100-CU steps. For Cochlear, we collect intraoperative ECAP data on odd electrodes using stimulation levels 190 to 230 CL, in 10-CL steps. For MED-EL, we collect intraoperative ECAP data on odd electrodes plus electrode 12 using stimulation levels 0 to 1200 CU, in 300-CU steps. Thus, the maximum number of points in the ECAP amplitude growth function was five for all three manufacturers.
For reporting purposes, present levels at the ECAP threshold were first converted from clinical levels to microamperes (μA) and ECAP thresholds were then determined via linear regression of the ECAP amplitude growth function for each manufacturer. Maximum ECAP amplitudes in microvolts (μV) were recorded from the intraoperative responses using similar across-subject maximum stimulation levels per the institution’s clinical protocol which is standardized for a given implant manufacturer. For Advanced Bionics, Cochlear, and MED-EL, the maximum stimulation level used for intraoperative ECAP testing is 1,216, 1,114, and 1,200 μA, respectively. The equivalent charge per phase for these levels and corresponding pulse durations was 38.9, 27.9, and 36.0 nC for Advanced Bionics, Cochlear, and MED-EL, respectively. ECAP amplitudes were reported for the maximum stimulation levels used. Postoperative audiograms were completed at the initial activation audiology appointment, approximately three weeks after surgery.
Preservation of postoperative low-frequency hearing was defined as having an air conduction audiometric threshold at 250 Hz ≤90 dB HL. This threshold was chosen because the target gain with acoustic amplification is theoretically achievable when figuring a half-gain rule for acoustic amplification and typical low-frequency gain limits for conventional hearing aids (range 40–45 dB). This frequency (250 Hz) was chosen for the following reasons: (a) it is the lowest frequency for which clinicians can verify hearing aid output for various prescriptive fitting targets and, thus, serves as a marker for functional residual hearing, and (b) acoustic hearing low-pass filtered at 250 Hz is the minimum bandwidth for which a significant additive benefit can be derived from the addition of acoustic hearing from either the implanted or non-implanted ear in adult cochlear implant recipients (Keidser et al, 2011; Zhang et al, 2013; Sheffield and Gifford, 2014; Sheffield et al, 2015). Although 90-dB HL was our criterion threshold for acoustic hearing preservation; this does not mean that we would provide acoustic amplification for frequencies with thresholds up to 90-dB HL for these individuals. Rather, the preservation of acoustic hearing in the implanted ear was chosen as a surrogate marker for cochlear structural preservation. Although we recognize that we cannot guarantee cochlear structural preservation, one could reasonably argue that structural preservation must be present—at least to some degree—for patients with residual acoustic hearing after CI.
Statistical Analysis
Analyses were performed with GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA) and SPSS Statistics version 25 (IBM, Armonk, NY). We first completed a linear mixed model test of interaction effects for the following four variables: manufacturer, electrode location (basal, middle, and apical), preoperative low-frequency pure-tone average (LFPTA), and postoperative LFPTA. There were no statistically significant four-way interactions among the aforementioned variables for both the ECAP threshold [F(254, 8) = 1.2, p = 0.44, ] and the ECAP amplitude [F(159, 8) = 0.3, p = 0.99, ]. In the absence of interaction effects, we analyzed electrode location and residual low-frequency data individually for both ECAP threshold and ECAP amplitude using multiple t-tests, without assuming a consistent standard deviation (SD) between the groups. Correction of alpha for multiple comparisons was conducted using the Holm–Sidak method, and the effect size was measured using Cohen’s d (d). Means and SDs for outcomes reported herein are representative of all included patients, whereas statistical comparisons of ECAP measurements reflect values for only those patients who had data available for the specified ECAP parameter. Nominal data were analyzed using a Fisher exact or chi-squared test. p values <0.05 were considered statistically significant.
RESULTS
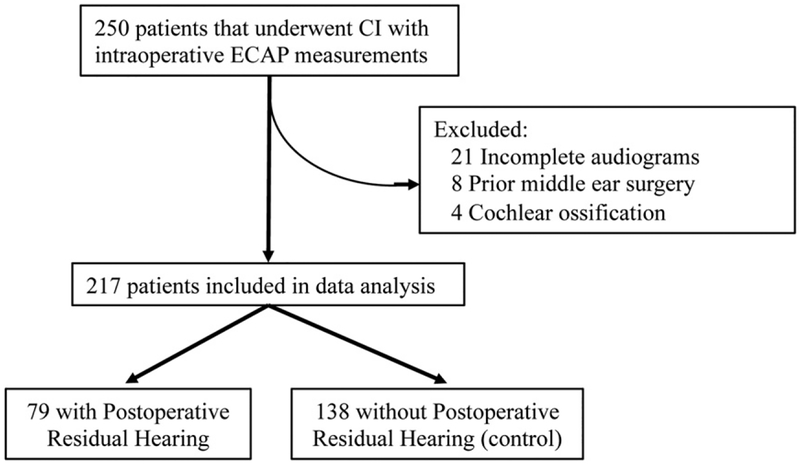
Two hundred fifty participants, with and without preoperative low-frequency acoustic hearing, who underwent CI with intraoperative ECAP measurements between 2011 and 2016 were identified. Patients were excluded for incomplete audiologic data (n = 21), history of prior middle-ear surgery (n = 8), and cochlear ossification (n = 4) (Figure 1). Ultimately, 217 participants were included in the analysis, 79 with postoperative residual low-frequency hearing and 138 control participants without postoperative residual acoustic hearing, as previously defined. Females accounted for 44.3% and 47.1% of the patients with and without postoperative residual low-frequency hearing, respectively (p = 0.8900) (Table 1). The median age was 66.1 and 66.5 years for patients with and without postoperative residual low-frequency hearing, respectively (p = 0.9193). Electrode insertion was conducted through one of the three surgical approaches: round window, extended round window, or cochleostomy; round window insertion was pursued if anatomy allowed. The cohort with postoperative residual low-frequency hearing had a larger portion of round window insertions than the cohort without postoperative residual low-frequency hearing (81.0% and 65.2%, respectively, p = 0.0097). The cohort without postoperative residual low-frequency hearing had a larger portion of cochleostomy approaches (21.7%) than the cohort with postoperative residual low-frequency hearing (8.9%, p = 0.0103). The distribution of surgeons between the two groups was not statistically different (analysis of variance, p = 0.687). The cohort with postoperative residual low-frequency hearing had an average postoperative LFPTA of 76.5 dB; specifically, 62.3, 75.8, and 91.2 dB at 125, 250, and 500 Hz, respectively. The cohort without postoperative residual low-frequency hearing had an average postoperative LFPTA of 100.6 dB; specifically, 90.7, 102.1, and 106.5 dB at 125, 250, and 500 Hz, respectively. Patients received implants from one of the three manufacturers: Advanced Bionics (Valencia, CA), Cochlear Americas (Englewood, CO), or MED-EL (Innsbruck, AT). The cohort with postoperative residual low-frequency acoustic hearing had a larger portion of MED-EL electrodes than the cohort without postoperative residual low-frequency hearing (53.2% and 37.0%, respectively, p = 0.0230). Notably, the preoperative LFPTAs between the three manufacturers were not statistically different (analysis of variance, p = 0.178).
Figure 1.
Study design.
Table 1.
Patient Demographics and Surgical Factors
| Total | Residual Hearing | No Residual Hearing | p Value | |
|---|---|---|---|---|
| n | 217 | 79 | 138 | - |
| Female (%) | 46.1 | 44.3 | 47.1 | 0.8900 |
| Age (years) | 66.4 | 66.1 | 66.5 | 0.9193 |
| Surgical approach (%) | ||||
| Round window | 71.4 | 81.0 | 65.2 | 0.0097 |
| Cochleostomy | 17.1 | 8.9 | 21.7 | 0.0103 |
| Extended round window | 11.5 | 10.1 | 13.0 | 0.5282 |
| Implant manufacturer (%) | ||||
| MED-EL | 42.9 | 53.2 | 37.0 | 0.0230 |
| Cochlear America | 37.8 | 29.1 | 42.8 | 0.0584 |
| Advanced Bionics | 19.4 | 17.7 | 20.3 | 0.7227 |
Note: Statistically significant p values are in bold.
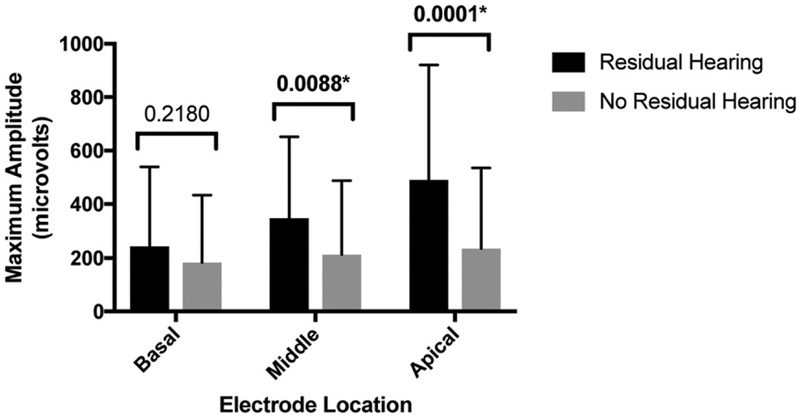
For all analyses, ECAP measurements were categorized by electrode location (apical, middle, or basal), and values at each electrode were averaged within each group. The average ECAP amplitude measurements (SD) for apical, middle, and basal electrodes were 490.73 μV (429.54 μV), 348.63 μV (303.36 μV), and 243.36 μV (296.54 μV), respectively, for the residual low-frequency hearing cohort, and 235.64 μV (300.172 μV), 213.23 μV (274.27 μV), and 183.13 μV (250.62 μV), respectively, for the non-residual low-frequency hearing cohort (Figure 2). For the apical and middle electrode ECAP amplitudes, there was a significant difference between groups with and without residual low-frequency hearing (p = 0.0001, d = 0.71 and p = 0.0088, d = 0.47, respectively), whereas the basal electrode ECAP maximum amplitudes were not different between groups (p = 0.2180, d = 0.22).
Figure 2.
ECAP maximum amplitude in patients with and without postoperative residual low-frequency hearing. Statistically significant p values are marked with asterisks (*).
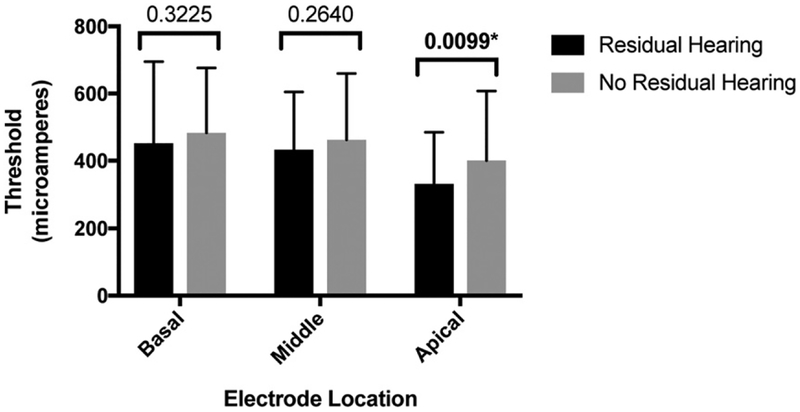
A similar analysis was conducted between the cohorts with and without residual low-frequency hearing for ECAP thresholds. The average ECAP thresholds measured intraoperatively for apical, middle, and basal electrodes were 330.95 μA (154.15 μA), 433.14 μA (172.12 μA), and 452.36 μA (242.64 μA), respectively, for the residual hearing cohort, and 400.58 μA (206.97 μA), 462.66 μA (197.12 μA), and 482.83 μA (193.44 μA), respectively, for the non-residual hearing cohort (Figure 3). For the apical region, ECAP thresholds were significantly lower in the residual low-frequency hearing group (p = 0.0099, d = 0.37), whereas there was no significant difference between groups for the middle and basal electrode regions (p = 0.2640, d = 0.16 and p = 0.3225, d = 0.14, respectively).
Figure 3.
ECAP threshold in patients with and without postoperative residual low-frequency hearing. Statistically significant p values are marked with asterisks (*).
Of note, a statistical analysis of patients divided into three groups ([a] patients without preoperative low-frequency hearing, [b] patients with preoperative low-frequency hearing who experienced preserved low-frequency hearing postoperatively, and [c] patients with preoperative low-frequency hearing who did not experience preserved low-frequency hearing postoperatively) was conducted with similar results as the abovementioned analysis. A comparison of the patients without preoperative low-frequency hearing to the patients with preoperative low-frequency hearing that preserved low-frequency hearing showed a statistically significant difference for ECAP maximum amplitude in the apical and middle regions (p = 0.0017 and p = 0.0119, respectively), but no statistically significant difference in the basal region (p = 0.1100). ECAP thresholds were not statistically significant between these two groups for apical, middle, or basal electrodes (p = 0.1230, p = 0.3296, and p = 0.2373, respectively).
DISCUSSION
The primary objective of this study was to evaluate the association between intraoperative ECAP measurements at the time of cochlear implant surgery and postoperative residual low-frequency acoustic hearing. The abovementioned findings support our hypothesis that patients with more robust intraoperative ECAP responses (e.g., lower ECAP thresholds and higher ECAP amplitudes) would have better rates of postoperative acoustic hearing preservation. Specifically, patients with postoperative residual low-frequency hearing did exhibit significantly larger maximum ECAP amplitudes for apical and middle electrodes and lower ECAP thresholds for apical electrodes during intraoperative ECAP testing. These findings were observed when comparing ECAP data for patients with considerable preoperative hearing (i.e., hearing preservation candidates) with both a group of patients who did not have viable preoperative hearing for preservation and hearing preservation candidates who ultimately did not have hearing preservation.
Previous studies provide some insight into the association between hearing preservation and ECAP amplitudes in the middle and apical electrodes. Maximum amplitudes may serve as a marker for neural health, as larger amplitudes are positively correlated with spiral ganglion cell density (Hall, 1990; Ramekers et al, 2014). Correspondingly, ECAP amplitudes were found to be consistently smaller after deafening in animal models (Shepherd and Javel, 1997; Agterberg et al, 2009). Our study did not reveal an association between ECAP amplitude in the basal electrodes and hearing preservation; this may be related to the tonotopic organization of the cochlea, as cochlear structures responsible for preserved low-frequency acoustic hearing are generally located at the cochlear apex. Furthermore, most patients included did not have preoperative acoustic hearing in the basal cochlea, and we theorize that all ECAP responses resulting from intracochlear electrical stimulation in the basal cochlea resulted from electroneural stimulation, and thus, we would not expect there to be differences between groups. By contrast, for patients with preoperative acoustic hearing in the apical cochlea, we hypothesized that a higher ECAP amplitude measured at the time of electrode placement would be an indicator of residual hearing, which could be inferred as resulting from an atraumatic surgical insertion. Although the present data seem to support this hypothesis, verification requires animal studies to document the degree of intracochlear insertion trauma, intraoperative ECAP amplitude, and subsequent histologic analysis.
The findings of this study, taken into context of previous reports, suggest that the intraoperative ECAP maximum amplitude, a marker for neural health, may function as an indicator of intraoperative neural injury. Future studies will search for a specific numerical value or cutoff of ECAP maximum amplitude at which hearing preservation surgery is most likely successful. Intraoperative ECAP measurements could eventually serve as immediate feedback for the surgeon regarding surgical technique and likelihood for acoustic hearing preservation; however, additional prospective studies are required before application in clinical practice.
ECAP thresholds, and their association with residual low-frequency hearing, are not completely understood. Lower ECAP thresholds have been associated with a shorter distance between the electrode and modiolus (Gordin et al, 2009; Davis et al, 2016). It follows that lower apical thresholds in our study would correlate with improved proximity to the modiolus, although it is not clear whether better perimodiolar placement would necessarily be related to higher rates of hearing preservation, unless perimodiolar placement reflected a complete scala tympani insertion, as scala tympani electrode location has been associated with increased rates of hearing preservation (O’Connell et al, 2016; 2017a,b). Further research and electrode imaging to verify scalar location are warranted.
Because of the nature of retrospective investigation, this study has inherent limitations, and thus, findings would benefit from confirmation through a controlled, prospective study. Specifically, a comparison of surgical approaches between the postoperative residual and non-residual hearing groups revealed a greater number of cochleostomy approaches in the non-residual hearing group. Although the cochleostomy approach has been associated with lower rates of hearing preservation than a round window approach (Wanna et al, 2018), it is possible that a cochleostomy was chosen more commonly for traditional cochlear implant candidates or in more challenging cases. In addition, a comparison of manufacturers revealed a greater number of MED-EL electrodes used in the residual low-frequency group, despite no statistically significant difference in preoperative LFPTAs across manufacturers; future prospective studies will focus on controlling for manufacturer and insertion approach; however, evaluation of hearing preservation rates were outside the scope of this study. Of note, pulse durations used for ECAP software are different among manufacturers. In this study, stimulation levels were converted into charge units, which were relatively comparable across manufacturers, although not identical. Although the ECAP stimulation and acquisition parameters are inherently different across the manufacturers, future research should attempt to equate stimulation levels in charge across the manufacturers, allowing for more accurate across-device comparison. Using current-generation software and hardware, with pulse durations of 32, 25, and 30 μsec per phase for AB, Cochlear, and MED-EL, respectively, one could set the upper stimulation level for intraoperative ECAP at 400 CU for AB, 240 CL for Cochlear, and 1100 CU for MED-EL, and this would equate the stimulation level in charge at ~33 nC per phase for each manufacturer.
The retrospective study design also limits the evaluation of potential confounders such as acute inflammation and surgical complications, which may occur between the time of the intraoperative ECAP measurements and postoperative audiologic testing. These events may impact audiologic outcomes in a manner that is not captured in this study. Last, this study was not designed as a prospective hearing preservation study to investigate hearing preservation rates or techniques at our center, as patients with and without preoperative low-frequency hearing were included. Thus, the conclusions drawn should be interpreted with caution until prospective studies investigating ECAP as a part of hearing preservation protocol are performed.
As a result of minimally traumatic insertion techniques and advances in electrode design, cochlear implant indications have expanded to include patients with residual acoustic hearing. Hearing preservation outcomes vary significantly and the status of residual low-frequency hearing is unknown until the first postoperative audiologic appointment. Intraoperative ECAP measurements may provide immediate insight to residual low-frequency hearing outcomes; patients with larger intraoperative ECAP maximum amplitudes and lower ECAP thresholds are more likely to have postoperative residual low-frequency acoustic hearing at their initial postoperative evaluation versus patients who are classical candidates or those who have low-frequency threshold shift. Future prospective studies will focus on clinical applications of intraoperative ECAP measurements and the potential use of ECAP values as real-time feedback for surgeons.
CONCLUSIONS
The present study demonstrates that patients with postoperative residual low-frequency acoustic hearing exhibited greater ECAP amplitudes for apical and middle electrodes and lower thresholds at the apical electrodes. This finding is attributed to the preservation of intracochlear neural substrate, namely, inner hair cells, with atraumatic electrode insertion and the resulting ECAP responses resulting from both electrophonic and electroneural stimulation. Indeed, the association between ECAP measurements and residual low-frequency hearing may represent a potential immediate feedback mechanism for postoperative outcomes that can be applied to all CIs. Additional animal studies could prove useful for verification of these data following surgical insertion, ECAP assessment, and subsequent histology.
Acknowledgments
Support for this research was provided by NIDCD R01 DC009404 and NIH NCATS UL1 TR000445.
Abbreviations:
- CI
cochlear implantation
- ECAP
electrically evoked compound action potential
- LFPTA
low-frequency pure-tone average
- SD
standard deviation
Footnotes
American Neurotology Society (ANS) oral presentation at Combined Otolaryngology Spring Meetings (COSM) 2018, National Harbor, MD.
René Gifford is on the Audiology Advisory Board for Advanced Bionics and Cochlear, and the Clinical Advisory Board for Frequency Therapeutics. David Haynes is a Consultant for Med-EL, Advanced Bionics, Stryker, and Cochlear.
REFERENCES
- Abbas PJ, Tejani VC, Scheperle RA, Brown CJ. (2017) Using neural response telemetry to monitor physiological responses to acoustic stimulation in hybrid cochlear implant users. Ear Hear 38:409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, van Dijk LM, de Groot JC, Klis SF. (2009) Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol 10:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Borland J, Bertschy MR. (1996) Electrically evoked whole nerve action potentials in Ineraid cochlear implant users: responses to different stimulating electrode configurations and comparison to psychophysical responses. J Speech Hear Res 39:453–467. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz BJ. (1998) Preliminary experience with neural response telemetry in the nucleus CI24M cochlear implant. Am J Otol 19:320–327. [PubMed] [Google Scholar]
- Brown CJ, Hughes ML, Luk B, Abbas PJ, Wolaver A, Gervais J. (2000) The relationship between EAP and EABR thresholds and levels used to program the nucleus 24 speech processor: data from adults. Ear Hear 21:151–163. [DOI] [PubMed] [Google Scholar]
- Bruce IA, Todt I. (2018) Hearing preservation cochlear implant surgery. Adv Otorhinolaryngol 81:66–73. [DOI] [PubMed] [Google Scholar]
- Buchner A, Schussler M, Battmer RD, Stover T, Lesinski-Schiedat A, Lenarz T. (2009) Impact of low-frequency hearing. Audiol Neurootol 1(14, Suppl):8–13. [DOI] [PubMed] [Google Scholar]
- Carlson ML, Driscoll CL, Gifford RH, Service GJ, Tombers NM, Hughes-Borst BJ, Neff BA, Beatty CW. (2011) Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol 32:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TJ, Zhang D, Gifford RH, Dawant BM, Labadie RF, Noble JH. (2016) Relationship between electrode-to-modiolus distance and current levels for adults with cochlear implants. Otol Neurotol 37:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries L, Scheperle R, Bierer JA. (2016) Assessing the electrode-neuron interface with the electrically evoked compound action potential, electrode position, and behavioral thresholds. J Assoc Res Otolaryngol 17:237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi AA, Polak M, He J, Telischi FF, Balkany TJ, Van De Water TR. (2005) Pattern of hearing loss in a rat model of cochlear implantation trauma. Otol Neurotol 26(3):442–447. [DOI] [PubMed] [Google Scholar]
- Franck KH, Norton SJ. (2001) Estimation of psychophysical levels using the electially evoked compound action protential measured with the neural response telemetry capabilities of Cochlear Corporation’s CI24M device. Ear Hear 22:289–299. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. (2016) Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: final outcomes. Laryngoscope 126:962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, Skarzynski H, Lorens A, Polak M, Driscoll CL, Roland P, Buchman CA. (2013) Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear 34:413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Davis TJ, Sunderhaus LW, Menapace C, Buck B, Crosson J, O’Neill L, Beiter A, Segel P. (2017) Combined electric and acoustic stimulation with hearing preservation: effect of cochlear implant low-frequency cutoff on speech understanding and perceived listening difficulty. Ear Hear 38: 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin A, Papsin B, James A, Gordon K. (2009) Evolution of cochlear implant arrays result in changes in behavioral and physiological responses in children. Otol Neurotol 30:908–915. [DOI] [PubMed] [Google Scholar]
- Hall RD. (1990) Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res 49:155–168. [DOI] [PubMed] [Google Scholar]
- Helbig S, Adel Y, Rader T, Stover T, Baumann U. (2016) Long-term hearing preservation outcomes after cochlear implantation for electric-acoustic stimulation. Otol Neurotol 37:e353–e359. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Brown CJ, Abbas PJ, Wolaver AA, Gervaise JP. (2000) Comparison of EAP thresholds with MAP levels in the nucleus 24 cochlear implant: data from children. Hear Hear 24:164–174. [DOI] [PubMed] [Google Scholar]
- Hunter JB, Gifford RH, Wanna GB, Labadie RF, Bennett ML, Haynes DS, Rivas A. (2016) Hearing preservation outcomes with a mid-scala electrode in cochlear implantation. Otol Neurotol 37: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidser G, Dillon H, Flax M, Ching T, Brewer S. (2011) The NAL-NL2 prescription procedure. Audiol Res 1:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JR, Abbas PJ, Brown CJ, Etler CP, O’Brien S, Kim LS. (2010) The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otol Neurotol 31:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JR, Tejani VD, Abbas PJ, Brown CJ. (2017) Intracochlear recording of acoustically and electrically evoked potentials in nucleus hybrid L24 cochlear implant users and their relationship to speech perception. Front Neurosci 11:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk DL, Yates GK. (1994) Evidence for electrically evoked travelling waves in the guinea pig cochlea. Hear Res 74:38–50. [DOI] [PubMed] [Google Scholar]
- Moteki H, Nishio SY, Miyagawa M, Tsukada K, Iwasaki S, Usami SI. (2017) Long-term results of hearing preservation cochlear implant surgery in patients with residual low frequency hearing. Acta Otolaryngol 137:516–521. [DOI] [PubMed] [Google Scholar]
- Nadol JB Jr, Young YS, Glynn RJ. (1989) Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol 98:411–416. [DOI] [PubMed] [Google Scholar]
- Nuttall AL, Ren T. (1995) Electromotile hearing: evidence from basilar membrane motion and otoacoustic emissions. Hear Res 92:170–177. [DOI] [PubMed] [Google Scholar]
- O’Connell BP, Holder JT, Dwyer RT, Gifford RH, Noble JH, Bennett ML, Rivas A, Wanna GB, Haynes DS, Labadie RF. (2017b) Intra- and postoperative electrocochleography may be predictive of final electrode position and postoperative hearing preservation. Front Neurosci 11:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell BP, Hunter JB, Haynes DS, Holder JT, Dedmon MM, Noble JH, Dawant BM, Wanna GB. (2017a) Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope 127:2352–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell BP, Hunter JB, Wanna GB. (2016) The importance of electrode location in cochlear implantation. Laryngoscope Invest Otol 1:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SF, Grolman W. (2014) Auditory-nerve responses to varied interphase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol 15:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Baumhoff P, Kral A. (2016) Cochlear implant stimulation of a hearing ear generates separate electrophonic and electroneural responses. J Neurosci 36:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz-Leyzac KC, Pfingst BE. (2018) Assessing the relationship between the electrically evoked compound action potential and speech recognition abilities in bilateral cochlear implant recipients. Ear Hear 39:344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyyedi M, Nadol JB Jr. (2014) Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol 35:1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield SW, Gifford RH. (2014) The benefits of bimodal hearing: effect of frequency region and acoustic bandwidth. Audiol Neurotol 19:151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield SW, Jahn K, Gifford RH. (2015) Preserved acoustic hearing in cochlear implantation improves speech perception. J Am Acad Audiol 26:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. (1997) Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res 108:112–144. [DOI] [PubMed] [Google Scholar]
- Turner C, Gantz BJ, Reiss L. (2008) Integration of acoustic and electrical hearing. J Rehabil Res Dev 45:769–778. [DOI] [PubMed] [Google Scholar]
- Wanna GB, O’Connell BP, Francis DO, Gifford RH, Hunter JB, Holder JT, Bennett ML, Rivas A, Labadie RF, Haynes DS. (2018) Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. Laryngoscope 128:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Mountain DC, Hubbard AE. (1995) Electrically evoked basilar membrane motion. J Acoust Soc Am 97: 3030–3041. [DOI] [PubMed] [Google Scholar]
- Zhang T, Spahr AJ, Dorman MF, Saoji A. (2013) Relationship between auditory function of nonimplanted ears and bimodal benefit. Ear Hear 34:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]