Abstract
Newly isolated yeasts from different Tunisian microhabitats, such as soil, milk, olive brine, vinegar, and from olive mill wastewater‐contaminated biotopes were extensively studied for their biochemical arsenal and morphological features, i.e. cell, ascospore, and lipid body morphology. All strains were classified into the Ascomycota phylum. However, they showed great functional diversity, including different morphological and biochemical features, lipid production ability, and fatty acid profiles. Accordingly, the strains were placed in three different groups: Group I, which includes Candida species; Group II (Pichia and related); and Group III (Kluyveromyces marxianus strain CC1). Group I and II were characterized by a high percentage of oleic acid (41.6–65.3% of total lipids) while in Group III, linoleic acid was the major fatty acid (37.2%). Members of Group I and II were further grouped into subgroups according to their fatty acid composition. Among the newly isolated strains, Pichia etchellsii BM1 was able to accumulate around 25% wt/wt lipid per dry cell mass and thus characterized as oleaginous. Some other strains, such as Candida metapsilosis strain EL2, C. parapsilosis strain LV2, C. pararugosa strain BM24, and K. marxianus strain CC1, which are able to produce extracellular lipases, may be of interest for specific environmental applications and/or for the production of novel lipases.
Keywords: Ascomycota, Ascospore, Fatty acid profiles, Lipid body, Oleaginous strains
Abbreviations
- ITS
internal transcribed spacer
- LB
lipid body
- NLM
nitrogen‐limiting medium
- OMW
olive mill wastewater
- SCO
single cell oil
1. Introduction
Microbial lipids or single cell oils (SCOs) have drawn much attention in the last decades due to their significant applications in food and chemical industries. With few exceptions (i.e. some obligate pathogens), the microorganisms are able to synthesize fatty acids but only few of them accumulate storage lipids mostly consisting of triacylglycerols. Storage lipids are synthesized under stress conditions (i.e. nutrient limitation) and deposited within special cytoplasmic organelles known as lipid particles, lipid bodies (LBs), or oil bodies 1, 2, 3. In yeast, these lipid particles constitute up to 70% of the total lipid content of the cell 4.
SCOs may serve as a renewable source of edible oil that could replace some expensive and rare oils/fats such as cocoa butter 5, 6 or fish oil containing polyunsaturated fatty acids of nutritional and pharmaceutical importance 1, 7, 8. Furthermore, several researchers have focused on the production of renewable oleochemicals from microbial oils, for instance, fuels, soaps, rubber, textiles, paints, plastics, detergents, surfactants, lubricants, cosmetics, and many other chemicals 9, 10, 11.
Among heterotrophic microorganisms, oleaginous yeasts are considered as the most appropriate organisms for lipid production 8, 12. Although several yeast species namely Rhodotorula, Rhodosporidium, Yarrowia, Candida, Cryptococcus, and Lipomyces are known as oleaginous 12, 13, 14, 15, the research for new yeast strains possessing specific biochemical features is of high biotechnological interest. Evolution guides the organisms toward optimal exploitation of their natural microhabitats and therefore particular microhabitats may accommodate exceptional strains.
The aim of this investigation was to isolate new yeast strains from specific Tunisian microhabitats and to study their biochemical behavior emphasizing on their ability to accumulate lipids. The newly isolated strains were identified according to their internal transcribed spacer (ITS) sequence similarities and their biochemical features. Vegetative cell and ascospore morphology were studied after specific staining. Extensive studies regarding LB formation and features, as well as lipid accumulation abilities and fatty acid composition were performed, concluding that some new isolates (i.e. Pichia etchellsii) were able of producing significant amounts of lipids rich in oleic acid.
2. Materials and methods
2.1. Samples collection
Samples were collected from different Tunisian microhabitats. Specifically, two soil samples from 5–10 cm depth were collected from fields in Sfax and oasis of Tozeur southern Tunisia and two samples of soil contaminated with olive mill wastewater (OMW; i.e. soil received OMW, sludge from OMW evaporating ponds) were obtained. A fresh OMW sample was collected from a traditional olive mill located in Sfax. Samples from home‐made vinegar, brine of naturally fermented black olives, and three milk types i.e. colostrum, goat milk, and cow milk were collected from small farms located in Sfax. Prior to use, milk samples were incubated for 2 days at room temperature to initiate spontaneous fermentation by indigenous microorganisms. All samples were stored at 4°C for a short time period or −20°C for longer time period.
2.2. Isolation of yeast strains
Sample fractions were suspended in sterilized Ringer solution (NaCl 0.9%, w/v in water), incubated at 30°C, under agitation at 150 rpm for 2 h and aliquots of 1 mL were withdrawn and serially diluted in sterilized Ringer solution. A volume of 100 μL from each dilution was plated out on Sabouraud‐agar medium containing (in g L−1) peptone (Accumix, Verna, India), 5; glucose (Merck, Darmstadt, Germany), 20; agar (Chemipharma, Tunis, Tunisia), 20; and chloramphenicol (Sigma, Steinheim, Germany), 0.05. pH was adjusted to 5.5 before autoclaving. The plates were incubated for 48 h at 30°C. Colonies with distinct morphological features such as color, shape, and size were picked and purified by repeatable streaking on yeast peptone glucose (YPG) agar medium containing (in g L−1) yeast extract (Conda, Madrid, Spain), 5; peptone, 5; glucose (Merck), 10; and agar (Chemipharma), 20. The pH was adjusted to 5.5. The purified isolates were stored on YPG agar plates at 4°C. For long‐term preservation, the strains were stored in 20% glycerol at −80°C.
2.3. Strain identification
The isolates were identified based on their ITS sequences. The ITS rDNA regions were PCR‐amplified using ITS1 and ITS4 primers (Forward ITS 1–5′ TCC GTA GGT GAA CCT GCG G 3′ and Reverse ITS 4‐ 5′ TCC TCC GCT TAT TGA TAT GC 3′) and were directly sequenced 16. The generated sequences were compared for their similarity with those of reference yeast strains (BLAST search). The phylogenetic tree was inferred using the Neighbor‐Joining method excluding positions with gaps 17. The optimal tree with the sum of branch length = 1.77803902 was shown. Evolutionary analyses were constructed with MEGA6 using the Maximum Composite Likelihood method 18.
2.4. Biochemical and physiological characterization
The ability of the newly isolated strains to assimilate different carbon and nitrogen compounds were determined according to Van der Walt and Yarrow 19, while their ability to ferment glucose, fructose, sucrose, galactose, xylose, maltose, and lactose was tested using the method described by Wickerham 20. The lipolytic activity of the isolates was determined following the method of Kouker and Jaeger 21 with slight modifications. Briefly, plates containing Rhodamine‐B agar medium (consisted of YPG agar supplemented with olive oil and Rhodamine B [Sigma] both at 1‰) were inoculated and incubated for 72 h at 30°C. The lipolytic activity was detected by irradiating the plates with UV light. Positive strains gave an orange fluorescent zone around the colonies.
The production of pseudomycelia or true mycelia was revealed using slides covered with a thin layer of PDA (Conda) and inoculated with a streak of yeast cells. Slides were then covered by a sterile coverslip, placed onto rods inside sterile Petri dishes, and incubated for 48 h. Microscopic observations of undisturbed colonies held at magnification 400x 22. Ascospore formation by the yeasts was investigated by culturing the strains in two different sporulation media containing potassium acetate (Sigma) or ethanol (Scharlau, Barcelona, Spain) as carbon sources 23.
2.5. Staining and microscopy
The isolated strains were screened for their lipogenic abilities using Sudan Black B staining technique 24. The cultures were performed in test tubes containing 5 mL of a nitrogen‐limiting medium (NLM) having the following composition (in g L−1): glucose, 30; (NH4)2SO4 (Carlo Erba, Rodano, Italy), 0.5; yeast extract, 0.5; KH2PO4 (Fluka, Steinheim, Germany), 12; Na2HPO4 (Fluka), 12; MgSO4.7H2O (Fluka), 1.5; CaCl2.2H2O (Carlo Erba), 0.1; MnSO4.5H2O (Fluka), 0.0001; CuSO4.5H2O (BDH, Poole, England), 0.0001; Co(NO3)3.3H2O (Merck), 0.0001; ZnSO4.7H2O (Merck), 0.001. The initial pH of the medium was 6 ± 0.1 after autoclaving. The tubes were inoculated with 100 μL of a midexponential preculture on Potato Dextrose Broth‐PDB (Conda) medium and incubated in a rotary shaker at 180 rpm and 28°C. After 72 h of incubation, Sudan Black stained smears from each culture were observed under optical microscopy on oil immersion (1000x) for the presence of blue‐colored globules within the cell. Yeast strains showing intracellular blue oily globules were selected for further quantitative analysis. Detailed morphological features of yeast LBs and ascospores were studied after staining with Nile red fluorescence dye (Sigma) as described by Arous et al. 3.
2.6. Comprehensive evaluation of the lipogenic ability of selected strains
2.6.1. Culture conditions
Selected yeast strains were cultivated in duplicate in 250 mL Erlenmeyer flasks containing 50 mL of the above‐described NLM. The initial pH of the medium was 6 ± 0.1 after autoclaving. After sterilization (at 121°C for 20 min), the flasks were inoculated with 1 mL of a midexponential preculture on PDB medium containing 4 × 108 cells. The cultures were incubated in a rotary shaker at 180 rpm and 28°C for 96 h.
2.6.2. Analytical methods
Flasks were periodically withdrawn from the incubator and cell mass was harvested by centrifugation at 15 000 rpm for 15 min, washed with distilled water, and dried in an oven at 80°C to a constant weight. Reducing sugars concentrations in the growth medium were determined by the DNS method according to Miller 25. pH was measured during growth by using a selective electrode. pH values remained in the range of 6.0–6.5 during all growth steps.
Total cell lipids were extracted according to Folch et al. 26 using chloroform:methanol 2:1 v/v as a solvent. Transesterification of lipids was performed according to the AFNOR 27 method. Fatty acid methyl esters were analyzed as described by Arous et al. 3.
2.7. Statistical analysis of data
Data were subjected to one‐way analysis of variance followed by a Bonferroni post hoc test using IBM SPSS Statistics 21 software package. The null hypothesis was rejected at significance level of p ≤ 0.05.
3. Results and discussion
3.1. Isolation and identification of yeast strains
Twenty‐seven yeast strains were isolated, i.e. 11 strains from fresh OMW and different OMW contaminated samples, four strains from milk samples, six strains from olive brine, two strains from vinegar, and four strains from soil samples. Among them, 14 strains (namely BM1, M1, M3, M31, EL1, EL2, EC28, V6, LV2, CC1, EP2, BM24, O2, and LC1R) were identified as potential oleaginous strains according to the Sudan Black B staining technique. Supporting information Fig. S1 shows photomicrographs (1000×) of smears of yeast strains that gave positive test results with the above‐mentioned staining technique.
Selected strains were identified on the basis of their ITS sequences, which were compared for their similarities with reference yeast strains by a BLAST search. From the phylogenetic analysis shown in Fig. 1, it was possible to discriminate three different groups namely Group I, which encompass Candida species; Group II, encompassing Pichia species; and Group III, which encompass a strain belonging to Kluyveromyces sp.
Figure 1.
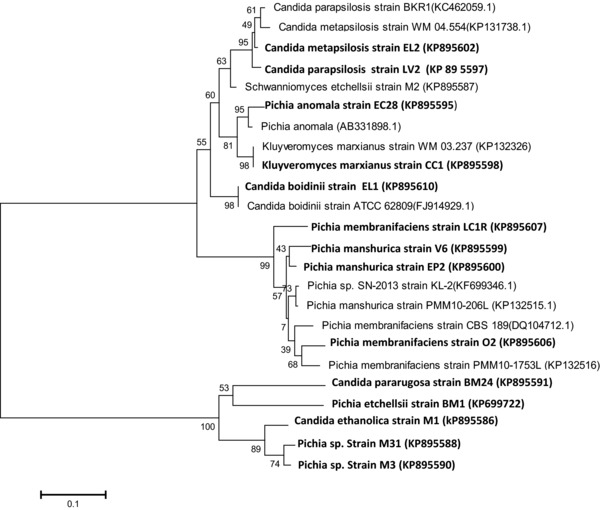
Phylogenic tree showing the relationships among the newly isolated yeast strains and related sequences collected from the Gene Bank.
3.2. Morphological and biochemical characterization of the new isolates
Morphological and biochemical features of the yeast isolates are shown in Table 1. Although all isolated strains were members of the Ascomycota phylum, only strains of Group II and III were able to sporulate. Concerning dimorphism, the standard procedure for mycelial growth on slide medium has long been used for taxonomic classification of yeasts. Morphological transition is of high importance in biotechnological applications, as single cells are easily manipulated in submerged cultures, while mycelial forms are preferred in solid‐state fermentation. Yeast‐mycelial dimorphism was already observed in a variety of yeasts including the genera Candida, Endomyces, Pichia, Saccharomyces, and Yarrowia in response to environmental conditions 28. In the case of Y. lipolytica, it has been reported that morphological transition from single cells to mycelia depends on several factors, such as the nature of the carbon and nitrogen sources, the presence of citrate or serum, the temperature and pH of culture media, as well as the dissolved oxygen concentration 22, 29, 30. In the present study, several Pichia strains were able to change between unicellular and filamentous growth. Yeast mycelia transition was also observed in some Candida species while Kluyveromyces marxianus strain CC1 was unable to produce hyphal or pseudohyphal forms.
Table 1.
Identification, biochemical and physiological characteristics of the newly isolated yeast strains: M1, M3, M31 were isolated from fresh OMW; EL1, EL2, EP2, EC28 were isolated from olive brine; BM24 and BM1 were isolated from OMW evaporating ponds; CC1 was isolated from colostrum; LV2 was isolated from cow milk; LC1R was isolated from goat milk; V6 was isolated from vinegar; O2 was isolated from soil; KL30, I6 and T1are reference strains identified as Candida succiphila, Pichia lachancei, and K. marxianus, respectively 33, 34, 35
| Candida | Pichia | Kluyveromyces | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | KL30 | M1 | EL1 | EL2 | LV2 | BM24 | I6 | M3 | M31 | O2 | EP2 | V6 | LC1R | EC28 | BM1 | T1 | CC1 |
| Assimilation | |||||||||||||||||
| d‐Glucose | + | ++ | +++ | ++ | ++ | ++ | + | ++ | +++ | + | +++ | ++ | +++ | ++ | ++ | + | +++ |
| Fructose | Nd | ++ | ++ | ++ | ++ | ++ | Nd | ++ | +++ | + | +++ | ++ | +++ | + | ++ | Nd | +++ |
| d‐Galactose | + | − | + | ++ | ++ | ++ | − | − | − | − | − | − | − | + | ++ | + | ++ |
| Lactose | Nd | − | + | + | ++ | + | − | − | + | − | − | − | − | + | − | + | ++ |
| Maltose | VW | − | + | ++ | − | + | + | + | + | − | − | + | + | + | + | − | + |
| Sucrose | + | ++ | + | ++ | ++ | ++ | + | ++ | ++ | + | ++ | ++ | + | ++ | ++ | + | +++ |
| l‐Arabinose | Nd | − | + | − | − | + | − | − | + | − | + | + | − | + | + | + | − |
| d‐Mannitol | + | − | + | ++ | − | ++ | Nd | − | − | − | − | − | − | + | + | Nd | + |
| Fermentation | |||||||||||||||||
| d‐Glucose | + | + | +++ | +++ | +++ | +++ | + | + | +++ | − | − | +++ | ‐ | + | + | + | +++ |
| Fructose | Nd | ++ | + | +++ | +++ | +++ | Nd | + | +++ | − | − | +++ | − | + | + | Nd | +++ |
| d‐Galactose | +/‐ | − | − | − | +++ | + | W | − | +++ | − | − | − | − | − | − | + | +++ |
| Lactose | − | − | − | − | ++ | − | Nd | − | − | − | − | − | − | − | − | + | +++ |
| Maltose | − | − | − | − | − | − | Nd | − | +++ | − | − | − | − | − | − | − | − |
| Sucrose | Nd | + | + | ++ | +++ | − | + | ‐ | +++ | − | − | + | − | − | − | + | +++ |
| d‐Xylose | Nd | − | − | − | − | − | Nd | − | − | − | − | − | − | − | − | Nd | − |
| Assimilation | |||||||||||||||||
| Urea | Nd | − | − | + | − | − | Nd | − | − | − | − | + | − | − | − | Nd | + |
| K NO3 | W/− | + | + | + | + | + | − | + | + | + | + | + | + | ++ | + | − | + |
| Yeast extract | + | + | + | + | + | + | + | + | + | + | ++ | ++ | ++ | + | + | + | ++ |
| l‐Lysine | − | − | − | + | − | − | + | − | − | + | − | + | − | − | − | + | + |
| (NH4)2SO4 | + | + | + | + | + | + | + | + | + | ++ | + | + | + | + | + | + | + |
| Filaments | − | + | + | − | − | − | − | + | + | − | + | + | + | − | − | Nd | − |
| Ascospores | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + |
| Lipolytic activity | Nd | − | − | + | + | + | Nd | +/− | +/− | +/− | +/− | +/− | +/− | +/− | − | Nd | + |
| Species name | |||||||||||||||||
| C. succiphila | C. ethanolica | C. boidinii | C. metapsilosis | C. parapsilosis | C. pararugosa | P. lachancei | Pichia sp. | Pichia sp. | P. membranifaciens | P. manshurica | P. manshurica | P. membranifaciens | P. anomala | P. etchellsii | K. marxianus | K. marxianus | |
| ITS sequence accession numbers | |||||||||||||||||
| ATCC46049 | KP895586 | KP895610 | KP895602 | KP895597 | KP895591 | ATCC201914 | KP895590 | KP895588 | KP895606 | KP895600 | KP895599 | KP895607 | KP895595 | KP699722 | ATCC22295 | KP895598 | |
Responses: + = positive; − = negative; +/− = mostly positive with some negative; W = weak; W/− = weak or negative; Nd = not determined; VW = very weak.
The assimilation and fermentation patterns of the isolates are discussed subsequently. In general, the strains did not ferment xylose while few of them were able to ferment maltose. In detail, concerning Group I, all strains were able to ferment glucose and fructose, but only C. pararugosa strain BM24 and C. parapsilosis strain LV2 were able to ferment galactose while C. boidinii strain EL1 and Candida metapsilosis strain EL2 were able to ferment sucrose. All strains, except for C. parapsilosis strain LV2, were able to assimilate glucose, fructose, sucrose, galactose, lactose, and maltose. Candida ethanolica strain M1 and C. parapsilosis strain LV2 failed to assimilate arabinose and mannitol. All strains were able to use KNO3, yeast extract, and (NH4)2SO4 as nitrogen sources. Candida metapsilosis strain EL2 was the only one able to utilize urea and l‐Lysine. Candida metapsilosis EL2, C. parapsilosis LV2, and C. pararugosa BM24 were the only strains possessing a strong lipolytic activity.
In Group II, the strains Pichia membranifaciens O2 and LC1R, and P. manshurica EP2 failed to ferment all tested carbon sources. However, these strains were able to grow in a range of carbon and nitrogen sources. Pichia sp. strain M31 was the most versatile to ferment and assimilate a variety of carbon sources such as sucrose, lactose, fructose, maltose, glucose, and arabinose, but was unable to assimilate urea and l‐lysine. Isolates P. etchellsii BM1 and P. anomala EC28 were able to ferment glucose and fructose and to assimilate a large variety of carbon sources, but, similarly to M31, were unable to assimilate urea and l‐lysine. Lipolytic activity was absent or weak in Pichia strains.
Kluyveromyces marxianus strain CC1, the only one of the Group III, was the most versatile to ferment and to assimilate a wide range of carbon sources and all the tested nitrogen sources. A strong lipolytic activity was observed in this strain.
Strains displaying an active lipase system are able to grow on fats, to accumulate and at the same time to modify the fatty acid profile of the initial substrate employed. Hence, lipase‐producing species could be used in the valorization of fatty materials in order to enhance their value 1. Additionally, it is of interest to assess the possibilities of manufacturing novel lipases by these strains that could be used in many branches of industry, such as dairy and fat industry as triglyceride modifiers, paper industry, as well as detergent enzyme 31. The above‐mentioned characteristics for the new isolates are rather similar to those of reference strains of Candida, Pichia, and Kluyveromyces 32, 33, 34.
3.3. LBs morphology of isolated strains
In eukaryotes, lipids are stored in the form of LBs, which can be easily visualized using Nile red staining technique. LBs’ number, shape, and localization have been determined from images at different focuses. Photomicrographs of Nile red stained cells of the 14 isolates during lipogenic phase (approximately at 72–96 h of growth) are presented in Fig. 2. It can be seen that LBs vary considerably in their morphology among genera and even among closely related species. Specifically, in strains of Group I, the number and the size of lipid droplets significantly varied between Candida species. The presence of one or two small LBs, one at each pole of the cell, was observed in C. ethanolica strain M1 (Fig. 2B) and C. boidinii strain EL1 (Fig. 2A). Nevertheless, many irregular LBs within the cytoplasm appeared in C. parapsilosis strain LV2 (Fig. 2D) and C. pararugosa strain BM24 (Fig. 2E), while C. metapsilosis strain EL2 (Fig. 2C) produced only one voluminous spherical LB close to the cell membrane. In strains of Group II, (Pichia species) cells exhibited lipid droplets comparable in shape, number, and localization since one or two spherical lipid droplets of different sizes were visualized at one of the two cell poles (Fig. 2F–M). Finally, cells of K. marxianus strain CC1 (Fig. 2N; Group III) produced an uncountable number of small LBs concentrated at one pole.
Figure 2.
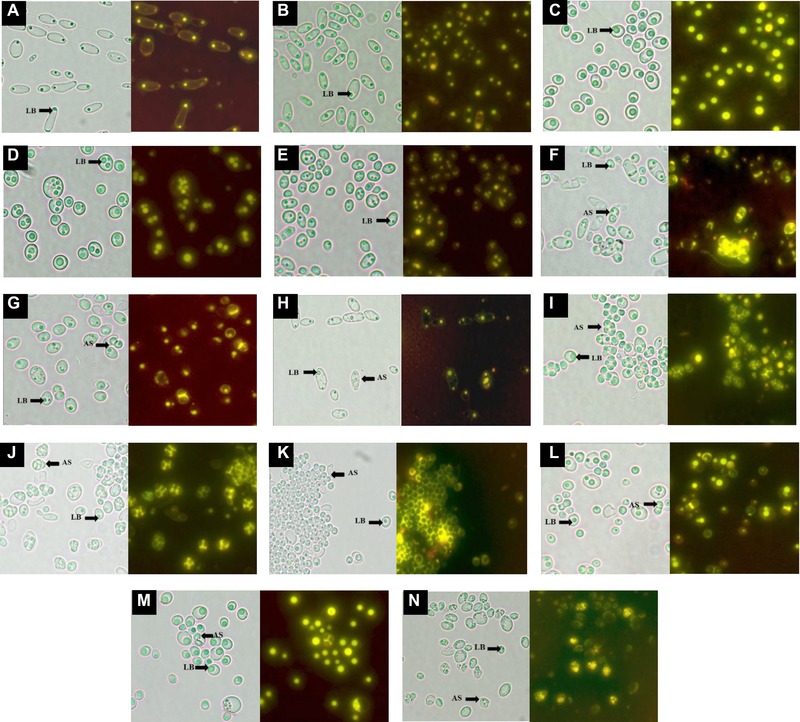
Morphological characteristics of yeasts LBs and ascospores during the lipogenic phase (72–96 h). Left panels: Transmitted light micrographs (1000x), right panels: fluorescence images of LBs and ascospores (AS) labeled with Nile red. Group I: (A) Candida boidinii EL1; (B) Candida ethanolica M1; (C) Candida metapsilosis EL2; (D) Candida parapsilosis LV2; (E) Candida pararugosa BM24; Group II: (F) Pichia sp. M3; (G) Pichia sp. M31; (H) Pichia manshurica EP2; (I) Pichia membranifaciens O2; (J) Pichia membranifaciens LC1R; (K) Pichia manshurica V6; (L) Pichia etchellsii BM1; (M) Pichia anomala EC28; Group III: (N) Kluyveromyces marxianus CC1. Culture conditions: growth on 250‐mL flasks in 50 mL of NLM; agitation rate 180 rpm; temperature 28 ± 1°C; initial glucose concentration 30 g L−1; initial pH 6 ± 0.1.
Nile red staining technique has been proven to be an excellent probe for visualizing lipid droplets of microorganisms 35. Nevertheless, it was hard to compare the correlation between lipid quantity and fluorescence signal by visual determination of the LBs size inside cells and the ratio of cells containing LBs 36. Thus, flow cytometry and lipophilic fluorescent probes should be combined in order to monitor lipid content within individual cells 37.
3.4. Ascospore morphology
Pichia species and K. marxianus strain CC1 were able to be reproduced both sexually (by sporulation) and asexually (by budding). Ascus formation occurs by conjugation between compatible cells, i.e. of a cell and its bud or between two independent cells. According to the literature, ascospores vary in number present in the ASCI, in morphology and in size depending on environmental and hereditary factors 23. In the present study, considerable variations in ascospore shape and number within species of the genus Pichia were revealed (Fig. 2F–M). Specifically, Pichia species produced one to four hat‐shaped to spherical ascospores within ASCI that tended to agglutinate after being released at maturity. Kluyveromyces marxianus strain CC1 (Fig. 2N) formed one to many globose to crescent‐shaped ascospores.
Many factors, including the nature of carbon and nitrogen source and the induction of the sporulation in buffers such as MES, piperazine‐N,N’‐bis 2‐ethanesulfonic acid, and MOPS, have been found to affect the process of sporulation and the number of spores per ascus 23. Ascosporogenesis has been studied in detail in the model yeast Saccharomyces cerevisiae. The complete absence of nitrogen and the presence of acetate, which is a nonfermentable carbon source, in the medium were shown to inhibit the budding process and to induce meiosis (ascosporogenesis stage) 38, 39. The sporulation of S. cerevisiae was accompanied by an extensive increase in dry weight due to the accumulation of intracellular carbohydrates 40. Similarly, in the present study, ascosporogenesis of Pichia species and K. marxianus strain CC1 proceeded after complete depletion of the nitrogen source in the growth medium (i.e. approx. in 72 h). However, the sexual reproductive stage was accompanied by the conversion of the carbon source into storage lipids that were accumulated mainly in ascospores. This phenomenon has been studied in detail by Arous et al. 3 for the oleaginous yeast Debaryomyces etchellsii (also known as P. etchellsii) grown in both batch and continuous cultures under nitrogen‐limiting conditions. The accumulated lipids may be used as energy reserve for ascospores germination as well as for surviving under unfavorable conditions.
Stained with Nile red, the growing and mature ascospores were surrounded by a thin fluorescent layer (Fig. 2F–N), suggesting the presence of 3–OH oxylipins. These compounds result from the conversion of certain lipids via incomplete β‐oxidation and may act as an adhesive responsible for the release and orderly reassembly of the ascospores 41, 42. The association of these oxylipins with ascospore aggregation may be similar to that observed in the yeast Dipodascus uninucleata 43.
3.5. Lipid accumulation
Growth kinetics of the selected strains were performed in NLM (containing 30 g L−1 glucose) and several parameters including cell mass yield, glucose consumption, and lipid content were estimated (Table 2). In all cases, growth of yeast strains was not accompanied by the production of organic acids, as insignificant pH changes were observed during all growth steps. Strains of Group I, especially Candida boidinii strain EL1, C. metapsilosis strain EL2, and C. parapsilosis strain LV2, yielded satisfactory biomass production. Percentage of lipids inside the yeast cells (YL/x) ranged from 8.5–12.9%, wt/wt. It seems, therefore, that under these culture conditions Candida strains accumulated low lipid quantities. Nevertheless, much higher lipid content has been previously reported for various Candida species 44, 45. Aggelis et al. 45 demonstrated that, in chemostat culture, Candida sp. accumulated about 40% of lipids. Similar results were obtained in a strain of C. curvata when grown in both batch and continuous cultures with xylose as carbon source. Lipid accumulation occurred in batch and continuous cultures when it reached a level of 49 and 37% of the biomass, respectively 46. Candida orthopsilosis and C. oleophila accumulated lipids up to 42.8 and 15.3% wt/wt, respectively 47, 48. On the other hand, some Candida species (such as C. pulcherrima) did not virtually accumulate lipids 47. It seems, hence, that Candida spp. compromises a heterogeneous group showing a considerable variation in lipid content among species. Higher lipid quantities were accumulated by most Pichia strains (YL/x up to 25.1%, wt/wt). The strain Wickerhamomyces anomalus EC28 formerly P. anomala showed remarkable biomass and lipid production (i.e. 8.1 g L−1 and 16.1%, wt/wt, respectively) after 72 h of fermentation and therefore is a candidate of interest for further studies. The strain BM1that was identified as P. etchellsii (also known as D. etchellsii) accumulated the highest levels of intracellular lipids (i.e. 25.1%, wt/wt). Similar results were reported for P. etchellsii in Arous et al. 3 working in both flask and bioreactor batch and continuous cultures. The other Pichia strains accumulated lipids in the range of 11.8–16.1% and produced low biomass. Similar results concerning lipid synthesis have been reported for P. membranifaciens LFMB8 and P. segobiensis DQ4091661 that were capable of producing around 15 and 24.6% of lipids per dry cell mass, respectively, when cultivated in nitrogen‐limited media 47, 49. However, in other reports, Pichia spp. has been shown to be able of producing much higher lipid quantities 50. Kluyveromyces marxianus strain CC1 (Group III) produced 6 g L−1 of biomass containing 12.9% wt/wt lipids. This amount is significantly higher than that determined by Fonseca et al. 51 in K. marxianus CBS 6556 (5.2% wt/wt lipids). These differences in lipid production abilities may be attributed to the specific physiological behavior of each microorganism or to the differences in culture conditions.
Table 2.
Characterization of the newly isolated yeast strains cultivated in flasks
| Groups | Species | Fermentation time (h) | x (g L−1) | YL/x(%, wt/wt) | S (g L−1) | Yx/S |
|---|---|---|---|---|---|---|
| I: Candida | C. ethanolica M1 | 72 | 5.6 ± 0.1 | 12.3 ± 0.5 | 8.6 ± 0.2 | 0.26 |
| 96 | 6.0 ± 0.5 | 12.9 ± 0.4 | 2.0 ± 0.1 | 0.21 | ||
| C. boidinii EL1 | 72 | 7.5 ± 0.2 | 8.5 ± 0.0 | 0.8 ± 0.2 | 0.26 | |
| 96 | 8.6 ± 0.9 | 9.5 ± 1.7 | 0.2 ± 0.0 | 0.29 | ||
| C. metapsilosis EL2 | 72 | 8.5 ± 0.2 | 10.9 ± 0.9 | 9.8 ± 0.5 | 0.42 | |
| 96 | 8.9 ± 0.3 | 12.9 ± 1.0 | 8.0 ± 0.1 | 0.40 | ||
| C. parapsilosis LV2 | 72 | 7.6 ± 0.5 | 8.6 ± 0.4 | 13.6 ± 0.1 | 0.46 | |
| 96 | 7.7 ± 0.3 | 11.4 ± 2.0 | 13.1 ± 0.5 | 0.46 | ||
| C. pararugosa BM24 | 72 | 4.6 ± 0.1 | 9.4 ± 0.2 | 22.1 ± 0.4 | 0.58 | |
| 96 | 5.2 ± 0.4 | 9.2 ± 0.3 | 20.0 ± 0.3 | 0.52 | ||
| II:Pichia | Strain M3 | 72 | 4.5 ± 0.2 | 16.1 ± 0.3 | 14.3 ± 0.4 | 0.28 |
| 96 | 4.3 ± 0.3 | 13.8 ± 0.9 | 9.6 ± 0.2 | 0.21 | ||
| Strain M31 | 72 | 4.3 ± 0.2 | 15.3 ± 0.7 | 10.5 ± 0.0 | 0.22 | |
| 96 | 4.0 ± 0.2 | 13.1 ± 0.4 | 6.4 ± 0.8 | 0.17 | ||
| P. membranifaciens O2 | 72 | 5.2 ± 0.3 | 11.0 ± 1.5 | 11.3 ± 0.8 | 0.28 | |
| 96 | 5.4 ± 0.3 | 11.8 ± 1.6 | 6.5 ± 0.3 | 0.23 | ||
| P. membranifaciens LC1R | 72 | 5.1 ± 0.3 | 14.6 ± 0.2 | 11.5 ± 0.1 | 0.28 | |
| 96 | 5.3 ± 0.3 | 11.9 ± 0.4 | 7.4 ± 0.3 | 0.24 | ||
| P. manshurica EP2 | 72 | 4.9 ± 0.2 | 11.8 ± 0.8 | 9.9 ± 0.4 | 0.25 | |
| 96 | 5.1 ± 0.3 | 10.9 ± 0.5 | 3.7 ± 1.0 | 0.19 | ||
| P. manshurica V6 | 72 | 3.8 ± 0.1 | 15.4 ± 0.6 | 13.2 ± 0.8 | 0.23 | |
| 96 | 3.6 ± 0.3 | 14.8 ± 0.7 | 9.1 ± 0.3 | 0.17 | ||
| P. anomala EC28 | 72 | 8.1 ± 0.2 | 16.1 ± 1.0 | 0.2 ± 0.0 | 0.27 | |
| 96 | 8.3 ± 0.3 | 13.4 ± 0.9 | 0.2 ± 0.0 | 0.28 | ||
| P. etchellsii BM1 | 72 | 4.9 ± 0.1 | 25.1 ± 0.2 | 15.3 ± 0.7 | 0.33 | |
| 96 | 4.9 ± 0.2 | 23.1 ± 0.5 | 11.4 ± 0.3 | 0.26 | ||
| II: Kluyveromyces | K. marxianus CC1 | 72 | 6.0 ± 0.3 | 12.7 ± 1.0 | 0.3 ± 0.0 | 0.20 |
| 96 | 5.7 ± 0.3 | 10.4 ± 1.3 | 0.3 ± 0.0 | 0.19 |
Representation of biomass (x, g L−1), remaining substrate (glucose‐ S, g L−1), lipid in dry biomass (L/x, %, w/w), and cell mass yield (g biomass/g substrate, Yx/S). Data are presented as mean values from duplicate experiments.
Culture conditions as in Fig. 2.
The total lipid content of yeasts varies widely among species of the same genera and even among strains belonging to the same species. According to the literature, the majority of the yeasts contain lipids around 7–15% of biomass dry weight, while few strains have the ability to accumulate more than 20% lipids of their dry weight.
3.6. Fatty acid composition of selected yeasts
Analysis of lipids synthesized during growth of all isolated strains showed that the most common and abundant fatty acids produced were palmitic (C16:0), palmitoleic (Δ9C16:1), stearic (C18:0), oleic (Δ9 C18:1), and linoleic (Δ9,12C18:2) acid, while medium chain (e.g. C12 and C14) or long‐chain (e.g. C20 and C22) fatty acids were produced in small quantities (Table 3). These results are in accordance with Kurtzman and Fell 28 and Chatzifragkou et al. 47, who noticed the predominance of C16 and C18 fatty acids and especially oleic acid in yeasts. This fatty acid composition is similar to that of oleaginous plants and could be perfectly used for biodiesel production 10, 52.
Table 3.
Fatty acid composition (%, wt/wt) of total lipids produced by the newly isolated yeast strains
| Relative fatty acid composition (% wt/wt)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Subgroups | Species | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | Othersb |
| I: Candida | 1 | C. pararugosa BM24 | 13.3 ± 0.2b1 | 14.6 ± 0.0b2 | 4.9 ± 0.1a3, b3 | 63.0 ± 0.1a4 | 1.7 ± 0.3c5 | 0.4 ± 0.1c6 | 2.2 ± 0.2 |
| C. metapsilosis EL2 | 18.1 ± 0.1a1 | 2.3 ± 1.1C2 | 9.0 ± 0.0a3 | 63.5 ± 0.7a4 | 3.9 ± 0.0b5 | 2.4 ± 0.1b6 | 0.8 ± 0.0 | ||
| C. parapsilosis LV2 | 18.3 ± 0.1a1 | 5.6 ± 0.0C2 | 5.1 ± 0.0 a3, b3 | 65.3 ± 0.0a4 | 3.8 ± 0.1b5 | 0.9 ± 0.0c6 | 0.9 ± 0.1 | ||
| 2 | C. ethanolica M1 | 9.8 ± 0.2c1 | 19.2 ± 0.3a2 | 2.2 ± 0.5b3 | 45.7 ± 0.2b4 | 14.7 ± 0.3a5 | 5.1 ± 0.1a6 | 3.3 ± 0.2 | |
| C. boidinii EL1 | 17.5 ± 0.7a1 | 12.7 ± 1.3b2 | 6.4 ± 1.0a3 | 43.7 ± 0.0C4 | 17.6 ± 0.8a5 | 0.3 ± 0.1c6 | 1.8 ± 0.2 | ||
| II: Pichia | 1 | P. membranifaciens O2 | 11.1 ± 0.2f1 | 14.3 ± 0.2e2 | 2.2 ± 0.3e3, f3, g3 | 46.0 ± 0.1f4 | 19.4 ± 0.1f5 | 4.8 ± 0.2e6 | 11.1 ± 0.2 |
| P. membranifaciens LC1R | 12.0 ± 0.3f1 | 11.3 ± 0.2g2 | 4.2 ± 0.1e3 | 44.7 ± 0.3f4 | 19.7 ± 0.0e5 | 5.4 ± 0.0d6 | 2.6 ± 0.0 | ||
| P. etchellsii BM1 | 17.4 ± 0.1e1 | 7.2 ± 0.1i2 | 4.6 ± 0.2e3 | 41.6 ± 0.0g4 | 26.8 ± 0.1d5 | 0.1 ± 0.1g6 | 17.4 ± 0.1 | ||
| 2 | sp.M3 | 9.5 ± 0.1g1 | 12.8 ± 0.3f2 | 3.6 ± 0.3e3 | 49.2 ± 0.4e4 | 16.7 ± 0.2g5 | 5.7 ± 0.0d6 | 2.5 ± 0.2 | |
| sp.M31 | 10.8 ± 0.2f1 | 16.0 ± 0.2d2 | 3.4 ± 0.1e3, f3 | 49.0 ± 0.3e4 | 12.2 ± 0.1h5 | 5.5 ± 0.0d6 | 3.1 ± 0.1 | ||
| P. manshurica EP2 | 9.9 ± 0.1g1 | 8.9 ± 0.1h2 | 7.5 ± 0.0d3 | 50.0 ± 0.0d4 | 13.2 ± 0.0g5, h5 | 5.4 ± 0.1d6 | 9.9 ± 0.1 | ||
| P. manshurica V6 | 11.6 ± 0.2 f1 | 16.8 ± 0.3d2 | 3.0 ± 0.1e3, f3 | 50.4 ± 0.5d4 | 10.8 ± 0.0i5 | 4.8 ± 0.0e6 | 2.6 ± 0.1 | ||
| P. anomala EC28 | 23.2 ± 0.1d1 | 4.1 ± 0.2j2 | 6.6 ± 0.2d3 | 48.8 ± 0.1e4 | 14.7 ± 0.4g5 | 1.5 ± 0.2f6 | 1.1 ± 0.1 | ||
| III. Kluyveromyces | K. marxianus CC1 | 20.1 ± 0.2 | 14.2 ± 0.2 | 3.9 ± 0.1 | 22.0 ± 0.5 | 37.2 ± 0.5 | 0.0 ± 0.1 | 2.6 ± 0.1 | |
Culture conditions as described in Fig. 2. Each experimental point is the mean value of at least two independent determinations. Letters refer to comparisons at vertical reading for each yeast species within the same group; numbers followed by different letters indicate statistically significant differences at p ≤ 0.05. The experimental data were treated according to Bonferroni post hoc test.
When maximum percentage of cellular lipids per dry cell mass was achieved.
Others: C10:0, C12:0, C14:0, C14:1. Additionally, in the case of P. etchellsii, a long‐chain fatty acid probably Δ4,7,10,13,16,19C22:6.
Kock and Botha 53 showed the heterogeneity of fatty acid composition even within the same species of yeasts. Accordingly, several researches dealt with the utilization of cellular fatty acid data for identification purposes before ribosomal sequencing 54. In the present study, remarkable differences in fatty acid profiles and unsaturated fatty acid index among the isolates were noticed. Based on cellular fatty acids composition, it was possible to discriminate different subgroups in the Groups previously described. Specifically, strains of Group I could split into two distinct Subgroups according to their lipid content in both oleic and linoleic acids. Subgroup 1, including strains that were characterized by a high percentage of oleic acid and the low percentage of linoleic acid (C. pararugosa strain BM24, C. metapsilosis strain EL2, and C. parapsilosis strain LV2) and Subgroup 2, with a low percentage of oleic acid and a high percentage of linoleic acid in their lipids (C. ethanolica strain M1 and C. boidinii strain EL1). The above‐mentioned differences in fatty acid composition between Subgroups 1 and 2 are indeed statistically significant at p ≤ 0.05. Additionally, C. ethanolica lipids contained alpha linolenic acid (C18:3) in nonnegligible percentages. Significant differences in fatty acid composition among Candida species have been reported by Aggelis et al. 45 and Viljoen et al. 55.
Similarly, strains of Group II belonging to Pichia species, might be divided into two Subgroups on the basis of their lipid content in both oleic and linoleic acids. Subgroup 1, which is characterized by a low percentage of oleic acid and a high percentage of linoleic acid (p < 0.05), included P. membranifaciens strain O2, P. membranifaciens strain LC1R, and P. etchellsii strain BM1. Conversely, Subgroup 2 may include Pichia sp. strain M3, Pichia sp. strain M31, P. manshurica strain EP2, P. manshurica strain V6, and P. anomala strain EC28, having significantly higher oleic acid and lower linoleic acid percentages (statistically significant at p ≤ 0.05) when compared to the other strains from Group II. Lipids synthesized by P. anomala EC28 additionally differed from those produced by the other strains of this subgroup due to the significantly higher percentage in palmitic acid and the lower percentage in palmitoleic acid (statistically significant at p ≤ 0.05). Pichia manshurica strains synthesized lipids with a comparable fatty acid composition, although lipids of the strain V6 contained palmitoleic acid in a significantly higher percentage whereas in those of the strain EP2 higher amount of linoleic acid was found (p < 0.05). Both strains of Pichia sp. M3 and M31 produced lipids having a quite similar fatty acid composition characterized by a relatively high percentage of oleic acid. Nevertheless, some differences were noticed, since strain M3 produced lipids with a significantly higher percentage of linoleic acid and lower percentage of palmitoleic acid than those of strain M31. Finally, the lipids of K. marxianus strain CC1 (Group III) were characterized by the presence of linoleic acid in very high percentage and by the lowest percentage of oleic acid (p < 0.05).
4. Concluding remarks
New yeast strains were isolated from various Tunisian microhabitats and characterized for their morphology and biochemical abilities of both taxonomical and biotechnological interest, enriching the biological material that is available in the Enzyme Engineering and Microbiology Laboratory of the University of Sfax. The ability of some strains to grow well on a wide spectrum of carbon and nitrogen sources, together with their competence to synthesize nonnegligible quantities of reserve lipids of similar fatty acid composition to that of vegetable oils currently used in the biodiesel manufacture, make them an interesting material for further studies. Specifically, P. etchellsii strain BM1 may offer an alternative source for SCO production and therefore this organism should be studied in detail. Some strains, such as C. metapsilosis strain EL2, C. parapsilosis strain LV2, C. pararugosa strain BM24, and K. marxianus strain CC1, which are able to produce extracellular lipases, may be of interest for specific environmental applications (i.e. in the treatment of fatty wastes) and/or for the production of novel lipases possessing particular properties.
Practical application
The study of new microbial strains, especially of those isolated from unusual microhabitats, may reveal interesting biological material for biotechnological applications. Here, we studied several important biochemical and morphological properties of both taxonomical and biotechnological interest of newly isolated yeast strains from various Tunisian microhabitats. The ability of some strains to grow on a wide spectrum of carbon and nitrogen sources, together with their competence to synthesize nonnegligible quantities of reserve lipids, makes them an interesting material for further studies. Pichia etchellsii strain BM1 particularly offers an alternative source for single cell oil production. Some other strains, such as Candida metapsilosis strain EL2, C. parapsilosis strain LV2, C. pararugosa strain BM24, and Kluyveromyces marxianus strain CC1, which are able to produce extracellular lipases, may be of interest for specific environmental applications and/or for the production of novel lipases.
Supporting information
Figure S1 Optical microscopy pictures (x1000) of yeast cells stained with Sudan Black B. Lipid bodies appeared as black particuls within the cells. (A) strain BM1, (B) strain M3, (C) strain M31, (D) strain O2, (E) strain LC1R, (F) strain EC28, (G) strain EP2, (H) strain V6, (I) strain BM24, (J) strain M1, (K) strain LV2, (L) strain EL1, (M) strain EL2, (N) strain CC1.
Acknowledgments
Financial support has been provided by (A) the project “Aristeia” that is implemented under the NSRF –“OPERATIONAL PROGRAMME EDUCATION AND LIFELONG LEARNING” and is co‐funded by the European Union (European Social Fund) and the Greek State (Ministry of Education and Religious Affairs ‐ Greek General Secretariat for Research and Technology) and (B) the Ministry of Higher Education and Scientific Research, Tunisia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have declared no conflict of interest.
5 References
- 1. Papanikolaou, S. , Aggelis, G. , Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Tech. 2011, 113, 1031–1051. [Google Scholar]
- 2. Donot, F. , Fontan, A. , Baccou, J. C. , Schorr‐Galindo, S. , Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohyd. Polym. 2012, 87, 951–962. [Google Scholar]
- 3. Arous, F. , Triantaphyllidou, I. E. , Mechichi, T. , Azabou, S. et al., Lipid accumulation in the new oleaginous yeast Debaryomyces etchellsii correlates with ascosporogenesis. Biomass Bioenerg. 2015, 80, 307–315. [Google Scholar]
- 4. Rattray, J. B. M. , Yeasts, in: Ratledge C. I., Wilkinson S. G. (Eds.), Microbial Lipids, Academic Press, London: 1988, pp. 555–697. [Google Scholar]
- 5. Beopoulos, A. , Cescut, J. , Haddouche, R. , Uribelarrea, J. et al., Yarrowia lipolytica as a model for bio‐oil production. Prog. Lipid Res. 2009, 48, 375–387. [DOI] [PubMed] [Google Scholar]
- 6. Papanikolaou, S. , Chevalot, I. , Komaitis, M. , Aggelis, G. et al., Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa‐butter substitute from industrial fats. Ant. Leeuw. Int. J. G. 2001, 80, 215–224. [DOI] [PubMed] [Google Scholar]
- 7. Bellou, S. , Baeshen, M. N. , Elazzazy, A. M. , Aggeli, D. et al., Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014a, 32, 1476–1493. [DOI] [PubMed] [Google Scholar]
- 8. Bellou, S. , Triantaphyllidou, I.‐E. , Aggeli, D. , Elazzazy, A. M. et al., Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotech. 2016, 37, 24–35. [DOI] [PubMed] [Google Scholar]
- 9. Gunstone, F. D. , Overview and market data, in: Gunstone F. D., Hamilton R. J. (Eds.), Oleochemical Manufacture and Applications, CRC Press, Taylor & Francis, USA: 2001, pp. 1–22. [Google Scholar]
- 10. Hill, K. , Höfer, R. , Natural fats and oils, in: Höfer R. (Ed.), Sustainable Solutions for Modern Economies, RSC Publishing, UK: 2009, pp. 167–228. [Google Scholar]
- 11. Steen, E. J. , Kang, Y. , Bokinsky, G. , Hu, Z. et al., Microbial production of fatty‐acid‐derived fuels and chemicals from plant biomass. Nature 2010, 463, 559–562. [DOI] [PubMed] [Google Scholar]
- 12. André, A. , Chatzifragkou, A. , Diamantopoulou, P. , Sarris, D. et al., Biotechnological conversions of bio‐diesel derived crude glycerol by Yarrowia lipolytica strains. Eng. Life Sci. 2009, 9, 468–478. [Google Scholar]
- 13. Amaretti, A. , Raimondi, S. , Sala, M. , Roncaglia, L. et al., Single cell oils of the cold‐adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb. Cell Fact. 2010, 9, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ageitos, J. M. , Vallejo, J. A. , Veiga‐Crespo, P. , Villa, T. G. , Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [DOI] [PubMed] [Google Scholar]
- 15. Parreira, T. M. , Freitas, C. , Reis, A. , Roseiro, J. et al., Carbon concentration and oxygen availability affect lipid and carotenoid production by carob pulp syrup‐grown Rhodosporidium toruloides NCYC 921. Eng. Life Sci. 2015, 15, 815–823. [Google Scholar]
- 16. White, T. J. , Bruns, T. , Lee, S. , Taylor, J. , Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, in: Innis M. A., Gelfland D. H., Sninsky J. J., White T. J. (Eds.), PCR Protocols: A Guide to Methods and Applications, Academic Press, San Diego: 1990, pp. 315–322. [Google Scholar]
- 17. Saitou, N. , Nei, M. , The neighbor‐joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [DOI] [PubMed] [Google Scholar]
- 18. Tamura, K. , Stecher, G. , Peterson, D. , Filipski, A. et al., MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van der Walt, J. P. , Yarrow, D. , Methods for the isolation, maintenance, classification and identification of yeasts, in: Kreger‐van Rij N. J. W. (Ed.), The Yeasts: A Taxonomic Study, Elsevier Science Publishers, Amsterdam: 1984, pp. 45–105. [Google Scholar]
- 20. Wickerham, L. J. , Taxonomy of yeasts. U. S. Dept. Agric.Tech. Bull. 1951, 1029, 1–56. [Google Scholar]
- 21. Kouker, G. , Jaeger, K. , Specific and sensitive plate assay for bacterial lipases. Appl. Environ. Microb. 1987, 53, 211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellou, S. , Makri, A. , Triantaphyllidou, I. E. , Papanikolaou, S. et al., Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology 2014, 160, 807–817. [DOI] [PubMed] [Google Scholar]
- 23. Miller, J. J. , Sporulation in Saccharomyces cerevisiae, in: Rose A. H., Harrison J. S. (Eds.), The Yeasts, Academic Press, New York: 1989, pp. 489–541. [Google Scholar]
- 24. Thakur, M. S. , Prapulla, S. G. , Karanth, N. G. , Estimation of intracellular lipids by the measurement of absorbance of yeast cells stained with Sudan Black B. Enzyme Microb. Tech. 1989, 11, 252–254. [Google Scholar]
- 25. Miller, G. L. , Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar]
- 26. Folch, J. , Lees, M. , Sloane‐Stanley, G. A. , Simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [PubMed] [Google Scholar]
- 27. AFNOR , Recueil des Normes Françaises des Corps Gras, Grains Oléagineux et Produits Dérives (3rd edition), Association Française pour normalization, Paris: 1984, pp. 95. [Google Scholar]
- 28. Kurtzman, C. P. , Fell, J. W. (Eds.), The Yeasts, a Taxonomic Study, Elsevier, Amsterdam: 1998. [Google Scholar]
- 29. Domínguez, A. , Fermiñán, E. , Gaillardin, C. , Yarrowia lipolytica: An organism amenable to genetic manipulation as a model for analyzing dimorphism in fungi, in: Ernst J. F., Schmidt A. (Eds.), Dimorphism in Human Pathogenic and Apathogenic Yeast, Karger, Farmington: 2000, pp. 151–172. [DOI] [PubMed] [Google Scholar]
- 30. Pérez‐Campo, F. M. , Domínguez, A. , Factors affecting the morphogenetic switch in Yarrowia lipolytica . Curr. Microbiol. 2001, 43, 429–433. [DOI] [PubMed] [Google Scholar]
- 31. Kim, M. , Kim, H. , Lee, J. , Thermostable lipase of Bacillus stearothermophilus: High‐level production, purification, and calcium‐dependent thermostability. Biosci. Biotechnol. Biochem. 2000, 64, 280–286. [DOI] [PubMed] [Google Scholar]
- 32. Van der Walt, J. P. , The emendation of the genus Kluyveromyces v. d. Walt. Antonie van Leeuwenhoek 1965, 31, 341–348. [DOI] [PubMed] [Google Scholar]
- 33. Lee, J. D. , Komagata, K. , Pichia cellobiosa, Candida cariosilignicola, and Candida succiphila, new species of methanol‐assimilating yeasts. Int. J. Syst. Bacteriol. 1980, 30, 514–519. [Google Scholar]
- 34. Phaff, H. J. , Starmer, W. T. , Kurtzman, C. P. D. , Pichia lachancei sp. nov., associated with several Hawaiian plant species. Int. J. Syst. Bacteriol. 1999, 49, 1295–1299. [DOI] [PubMed] [Google Scholar]
- 35. Greenspan, P. , Fowler, S. D. , Spectrofluorometric studies of the lipid probe, Nile red. J. Lipid Res. 1985, 26, 781–789. [PubMed] [Google Scholar]
- 36. Cooksey, K. E. , Guckert, J. B. , Williams, S. A. , Callis, P. R. , Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J. Microbiol. Meth. 1987, 6, 333–345. [Google Scholar]
- 37. Guzmàn, H. M. , Valido, A. J. , Duarte, L. C. , Presmanes, K. F. , Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquacult. Int. 2010, 18, 189–199. [Google Scholar]
- 38. Croes, A. F. , Induction of meiosis in yeast. I. Timing of cytological and biochemical events. Planta 1967, 76, 209–226. [DOI] [PubMed] [Google Scholar]
- 39. Esposito, R. E. , Klapholz, S. , Meiosis and ascospore development, in: Strathern J. N., Jones E. W., Broach J. R. (Eds.), The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance, Cold Spring Harbor Laboratory Press, New York: 1981, pp. 211–287. [Google Scholar]
- 40. Roth, R. , Carbohydrate accumulation during the sporulation of yeast. J. Bacteriol. 1970, 101, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Venter, P. , Kock, J. L. , Kumar, G. S. , Botha, A. et al., Production of 3R‐hydroxy‐polyenoic fatty acids by the yeast Dipodascopsis uninucleata . Lipids 1997, 32, 1277–1283. [DOI] [PubMed] [Google Scholar]
- 42. Kock, J. L. F. , Van Wyk, P. W. J. , Venter, P. , Coetzee, D. J. et al., An acetylsalicylic acid‐sensitive aggregation phenomenon in Dipodascopsis uninucleata . Antonie van Leeuwenhoek 1999, 75, 261–266. [DOI] [PubMed] [Google Scholar]
- 43. Smith, D. P. , Kock, J. L. F. , Van Wyk, P. W. J. , Pohl, D. J. et al., Oxylipins and ascospore morphology in the ascomycetous yeast genus Dipodascus . Antonie van Leeuwenhoek 2003, 83, 317–325. [DOI] [PubMed] [Google Scholar]
- 44. Ratledge, C. , Microbial fats and oils: An assessment of their commercial potential. Prog. Ind. M. 1982, 16, 119–206. [Google Scholar]
- 45. Aggelis, G. , Stathas, D. , Tavoularis, N. , Komaitis, M. , Composition of lipids produced by some strains of Candida species. Production of single‐cell oil in a chemostat culture. Folia Microbiol. 1996, 41, 299–302. [Google Scholar]
- 46. Evans, C. T. , Ratledge, C. , A comparison of the oleaginous yeast Candida curvata, grown on different carbon sources in continuous and batch culture. Lipids 1983, 18, 623–629. [DOI] [PubMed] [Google Scholar]
- 47. Chatzifragkou, A. , Makri, A. , Belka, A. , Bellou, S. et al., Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar]
- 48. Kanti, A. , Sukara, E. , Kadarusman, L. , Sukarno, N. et al., Indonesian oleaginous yeasts isolated from Piper betle and P. nigrum. Mycosphere 2013, 4, 1015–1026. [Google Scholar]
- 49. Schulze, I. , Hansen, S. , Großhans, S. , Rudszuck, T. et al., Characterization of newly isolated oleaginous yeasts—Cryptococcus podzolicus, Trichosporon porosum and Pichia segobiensis . AMB Express 2014, 4, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kurtzman, C. P. , Fell, J. W. , Boekhout, T. (Eds.), The Yeasts, a Taxonomic Study, Elsevier, Amsterdam: 2011. [Google Scholar]
- 51. Fonseca, G. G. , Gombert, A. K. , Heinzle, E. , Wittmann, C. , Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res. 2007, 7, 422–435. [DOI] [PubMed] [Google Scholar]
- 52. Li, M. , Liu, G. L. , Chi, Z. , Chi, Z. M. , Single cell oil production from hydrolysate of cassava starch by marine‐derived yeast Rhodotorula mucilaginosa TJY15a. Biomass Bioenerg. 2010, 34, 101–107. [Google Scholar]
- 53. Kock, J. L. F. , Botha, A. , Fatty acids in fungal taxonomy, in: Frisvad J. C., Bridge P. D., Arora D. K. (Eds.), Chemical Fungal Taxonomy, Marcel Dekker, New York: 1998, pp. 219–246. [Google Scholar]
- 54. Kutty, S. N. , Philip, R. , Marine yeasts—A review. Yeast 2008, 25, 465–483. [DOI] [PubMed] [Google Scholar]
- 55. Viljoen, B. C. , Kock, J. L. F. , Muller, H. B. , Lategan, P. M. , Long‐chain fatty acid compositions of some asporogenous yeasts and their respective ascosporogenous states. J. Gen. Microbiol. 1987, 133, 1019–1022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Optical microscopy pictures (x1000) of yeast cells stained with Sudan Black B. Lipid bodies appeared as black particuls within the cells. (A) strain BM1, (B) strain M3, (C) strain M31, (D) strain O2, (E) strain LC1R, (F) strain EC28, (G) strain EP2, (H) strain V6, (I) strain BM24, (J) strain M1, (K) strain LV2, (L) strain EL1, (M) strain EL2, (N) strain CC1.


