Abstract
Schizochytrium sp. AB‐610 accumulates relatively higher amount of DHA‐rich lipid in the cells, and it was found that DHA yield was closely related to the cell morphology and pH value during fermentation period. DHA production from Schizochytrium sp. AB‐610 in fed‐batch fermentation was investigated and four growth stages were clarified as lag stage, balanced growth stage, lipid accumulation stage, and lipid turnover stage, based on the morphologic observation and key parameters changes. Then a simple strategy of two‐stage pH control was developed, in which pH 7.0 was kept until 12 h after the end of balanced growth stage, and then shifted to 5.0 for the rest period in fermentation. A maximal DHA production of 11.44g/L was achieved. This approach has advantage of easy scaling up for industrial DHA fermentation from Schizochytrium sp. cells.
Keywords: Docosahexaenoic acid, Fed‐batch fermentation, Morphological observation, pH control, Schizochytrium sp
Abbreviations
- DHA
docosahexaenoic acid
- DCW
dry cell weight
- FA
fatty acid
- FAS
fatty acid synthase
- FLB
full of lipid body
- LSCM
laser scanning confocal microscope
- PKS
polyketide synthase
- SEM
scanning electron microscope
1. Introduction
Schizochytrium sp., a thraustochytrid in the kingdom of Stramenopila, is an algae‐like microorganism 1, 2. It was first isolated and described by Goldstein and Belsky in 1964 3. DHA‐rich lipids produced by Schizochytrium sp. are concerned to be more appropriate than fish oils for the use, because fish oils, as the traditional commercial sources of DHA, have disadvantages such as high processing cost, seasonal variation of oil composition, declining fish stocks, marine pollution, and poor oxidative stability 4. Oil produced by Schizochytrium sp. is up to over 50% of cell weight, in which over 35% of total fatty acids (FAs) are DHA 5. The application of Schizochytrium sp. to produce DHA by industrial fermentation became more and more attractive.
Previous researches on Schizochytrium sp. DHA synthesis generally include screening high‐yield DHA strains 6, 7, trying different carbon and/or nitrogen sources 8, 9, 10, modulating culture conditions 11, 12, 13, optimizing fermentation parameters 14, 15 and adjusting fermentation process 10, 16, 17. Only a few studies focused on the relation of morphological characters to the Schizochytrium sp. DHA fermentation. An electron microscopic analysis employing sample preparation by high‐pressure freeze substitution was employed to explore the structure of DHA‐rich oil within Schizochytrium sp. 18. The relations between the lipid synthesis and lipid‐body formation in the culture of synchronous zoospore were described by light microscopy as well as electron microscopy using freeze substitution technique 19.
The pH value is one of the key parameters in fermentation. It involves in cell growth by affecting nutrients absorption through alternation of cell membrane charge. It was also essential to the metabolites production formation by affecting enzyme activities, which determine the metabolic direction 20. During fermentation period, pH keeps changing as the consumption of substrates and excretion of metabolites. Reports showed that pH condition was critical to lipid accumulation in Schizochytrium sp. 11, 21. According to the previous researches of other product fermentations, the optimal pH condition for cell growth and product formation are usually different 20, 22, 23. This inconsistent optimal pH condition may also occur during DHA production by Schizochytrium sp. A research investigating pH shift strategy in Schizochytrium sp. fermentation is needed for obtaining higher DHA yield.
In the present work, time course of five key parameters in the Schizochytrium sp. AB‐610 fermentation process (dry cell weight (DCW), glucose, nitrogen, lipid, and DHA) were investigated. In addition, cell growth features and lipid body development were observed by scanning electron microscopic (SEM) as well as laser scanning confocal microscope (LSCM) using Nile red staining. The relations between the lipids synthesis and lipid‐body formation were discussed. A two‐stage pH control strategy was further developed based on the observation of balanced growth stage and lipid accumulation stage. These results provided useful information for the industrial fermentation process of Schizochytrium sp. producing DHA‐rich microbial lipids.
2. Materials and methods
2.1. Microbial strain
The strain Schizochytrium sp. AB‐610 was an ARTP‐Bleomycin mutant from Schizochytrium sp. S31 (ATCC 20888) and preserved in our laboratory. The cells were maintained on slant culture with By+ medium contained (g/L): glucose, 5; peptone, 1; yeast extract, 1 and agar, 20 in artificial seawater, and sub‐cultured every 2 months.
2.2. Conditions of fed‐batch fermentation
The seed culture medium contained (g/L): glucose, 30; tryptone, 10; yeast extract, 5; monosodium glutamate, 5; KH2PO4, 2.5; MgSO4, 1.2; Na2SO4, 12.8; CaCl2, 0.4; Crystal Sea, 15. Initial fermentation medium contained (g/L): glucose, 100; tryptone, 5.6; monosodium glutamate, 20; KH2PO4, 2.5; MgSO4, 7.2; Na2SO4, 12.8; CaCl2, 0.4; Crystal Sea, 17.5. The media were autoclaved at 115°C for 20 min. Glucose was autoclaved separately. Vitamin solution contained (mg/L): VB1, 100; VB6, 100; VB12, 1. It was filter‐sterilized through a 0.22 μm filter and added after sterilization. The stored cells were inoculated into a 250‐ml flask with 50 ml seed culture medium and cultured at 28°C, 200 rpm for 48 h. After two generations of cultivation, the seed culture was inoculated to initial fermentation medium.
Fed‐batch fermentations were carried out in a 7.5 L NBS Bioflo 110 fermentor (USA) with a working volume of 3L. The inoculum volume was 10% (v/v) of initial fermentation medium. When glucose was consumed to lower than 15 g/L in broth during the fermentation process, certain amount of glucose stock solution (500 g/L) was added to the level of 40–50 g/L.
During the fermentation process, the cultivation temperature was kept at 28°C. In two‐stage pH control experiments and single constant pH experiments, pH was adjusted by HNO3 solution (8 mol/L) and NaOH solution (8 mol/L). The agitation speed and airflow rate were set at 400 rpm and 3 v/v min, respectively.
2.3. Assay of DCW, lipid, FA compositions, glucose, and nitrogen
A sample of 25 mL fermentation broth was taken every 12 h to measure the DCW, lipid yield, FA compositions, residual glucose, and ammonia nitrogen. The fermentation broth was centrifuged at 8000 × g for 15 min. The cell pellet was then washed twice with distillated water, and dried at 60°C to constant weight to measure the DCW. The cells were disrupted by incubating the mixture of 1 g of dry biomass and 8 mL of HCl (6 mol/L) in hot water bath at 75°C for 30 min. Lipids were extracted with 20 mL n‐hexane for three to four times and then dried in a rotary vacuum evaporator. The FAs in lipids were extracted and methylated according to 24. The fatty acid methyl esters (FAMEs) samples were analyzed by GC (GC‐2010, Shimadzu, Japan), equipped with an INNOWAX capillary column (30 m × 0.32 mm × 0.25 μm, Agilent, USA) and a flame ionization detector set at 250°C. The injector was maintained at 250°C with an inject volume of 1 μL. The column was kept at 130°C for 2 min, and rose at a rate of 10°C/min to 180°C, then rose at a rate of 4°C/min to 240°C, and kept at 240°C for 8 min. Nitrogen was used as carrier gas. FAs were identified through comparison with 40‐component FAME mix standard (NU‐CHEK, USA). The quantities of individual FAMEs were estimated using nonadecanoic acid (C19:0) as internal standard. Glucose was measured enzymatically using a bioanalyzer (SBA‐40C, Shandong Academy of Sciences, China) simultaneously. Ammonia nitrogen in the broth was assayed by formol number.
2.4. Preparation for scanning electron microscopic (SEM) and laser scanning confocal microscopic (LSCM) observation
For SEM observation, samples were fixed with 1% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.2), and post fixed with 1% OsO4 in 0.1 mol/L phosphate buffer (pH 7.2). Fixed samples were dehydrated through gradient ethanol and processed in isoamyl acetate and dried by critical CO2 method. Finally, the samples were fixed on SEM support, and then coated with gold using a sputter coater (SCD005, BAL‐TEC, Czech Republic) before observed under scanning electron microscope (Quanta 200, FEI, Holland).
For LSCM observation, cells were diluted to appropriate concentration, and 500 μL of Dimethyl sulfoxide and 30 μL 0.75 g/mL of Nile red solution were added to 3 mL of above diluent, stained at 37°C water bath for 30 min under dark condition. Samples were then observed via a LSCM (C2 confocal microscope system, Nikon, Japan) for differential interference contrast and fluorescence images.
3. Results and discussion
3.1. Clarification of four stages in fed‐batch fermentation of Schizochytrium sp. AB‐610 based on the cellular observation and key parameters analysis
After inoculating seed culture into fermentation medium in fed‐batch culture, cells were observed every 12 h with SEM to clarify the stages of morphological changes. The initial pH of fermentation medium was 5.35, and it was not readjusted during the fermentation period. According to key fermentation parameters (Fig. 1) and cell morphologies (Fig. 2), four stages were clarified as: Lag stage from 0 to 24 h, balanced growth stage from 24 to 48 h, lipid accumulation stage from 48 to 96 h, and lipid turnover stage from 96 to 120 h.
Figure 1.
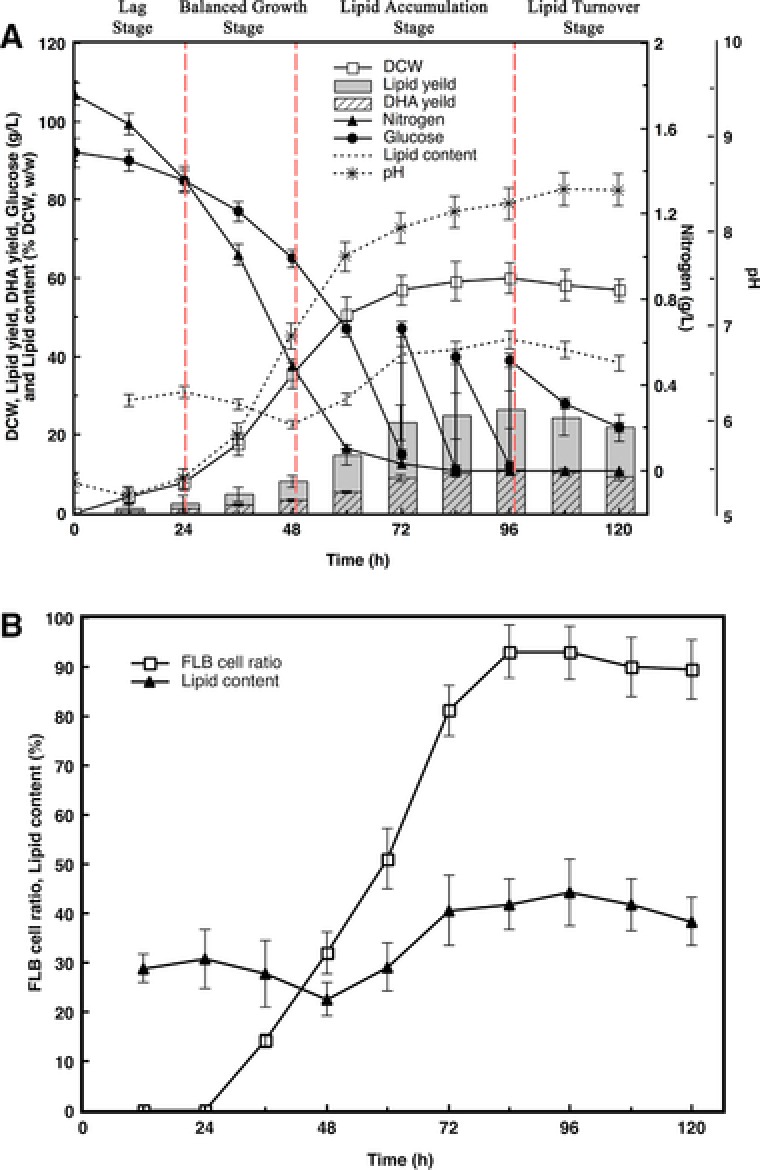
Time course of key parameters in fed‐batch fermentation by Schizochytrium sp. AB‐610. (A): Clarification of four stages. Trends of nitrogen and glucose consumption, DCW, lipid yield, lipid content, DHA yield, and pH changes. (B): Changes of lipid body morphology during lipid accumulation. Trends of lipid accumulation and FLB cell ratio.
Figure 2.
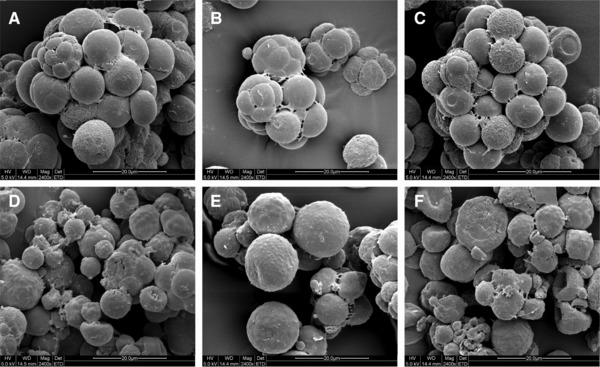
Changes of cell morphology in fed‐batch fermentation by Schizochytrium sp. AB‐610 detected by SEM. Cells were cultured for 0 h (a), 24 h (b), 48 h (c), 72 h (d), 96 h (e), and 120 h (f). Scale bar = 20 μm.
As Fig. 1A showed, Schizochytrium sp. AB‐610 biomass increased slowly in lag stage, for cells still adapted themselves to the new growth condition. Cells showed little sign of growth or proliferation from SEM image at this stage (Fig. 2A). Glucose and nitrogen were consumed to synthesize RNA, enzymes, and other molecules for the preparation of subsequent cells division and growth. The pH change was not obvious. Adequate nitrogen and glucose supply supported the cell rapid growth in the balanced growth stage, where the specific growth rate μ raised to 0.0635 h−1. At this stage, cells proliferated by successive binary vegetative cell divisions (Fig. 2B) and continued four or five times, consequently a primitive single cell became a cluster of 16 or 32 cells. The size of the vegetative cells was relatively uniform and became smaller (from 10 μm diameter (24 h) to 6 μm diam. (48 h)). As shown in Fig. 1A, lipid accumulation commenced from 24 h as the biomass increased while significant amount of nitrogen was still available in culture medium, but the lipid content in biomass rose since 48 h. From 48 to 96 h, the lipid yield increased from 8.02 to 26.54 g/L, while the DCW increased from 35.45 to 59.96 g/L, indicating that 75.56% of biomass increasing was due to the increased lipid content. The lipid accumulation rate increased from 0.235g/L/h in the balanced growth stage to 0.386 g/L/h in the lipid accumulation stage, and the total lipid reached 44.26% (w/w) of DCW as maximum at the end of the lipid accumulation stage. As shown in Fig. 2D, E, vegetative cells during lipid accumulation stage increased in sizes (10–16 μm diameter) and matured as zoosporangia. Most zoospores grew into vegetative cells and accumulated lipids, while only a small portion became zoosporangia. The pH was kept constant increasing during balanced growth stage and lipid accumulation stage, contributed to the fast consumption of nitrogen and rapid secret of metabolic waste. Since 96 h, fermentation ran into the lipid turnover stage, in which lipid contents decreased through oxygen‐involved β‐oxidation. Cells surface wrinkled as some of the lipids inside were consumed. When the fermentation process was near the end, cells were getting aged and cell activity declined. Debris from cell lysis appeared finally (Fig. 2F). The pH value at this stage showed a slightly decrease, possibly due to the release of organic acids from the cells into fermentation broth.
It was reported that two kinds of proliferation manners have been observed in Schizochytrium life cycle: 1: development of zoosporangium and release of zoospores in the early period and followed by 2: successive binary cell divisions and formation of a cell cluster 25. In our fed‐batch fermentation process, however, binary cell divisions occurred mainly at balanced growth stage, while zoosporangia appeared at lipid accumulation stage. It is possible that culture environmental variations contributed to different cell proliferation manners. As the proliferation manner of Schizochytrium sp. changed with the fermentation process, the fermentation stages can be generically judged by the main proliferation manners of cells. Different from other oleaginous microorganisms that lipid accumulation occurs when lack of nitrogen 26, 27, 28, Schizochytrium sp. accumulated lipid before nitrogen exhausted, suggesting that in Schizochytrium sp. the lipid biosynthesis rate is higher than growth rate as cells are quick enough to accumulate lipid before they convert it into new cells. This characteristic of growth‐associated lipid accumulation process was also found in other reports on thraustochytrids 29, 30 and oleaginous yeasts 31.
Clarification of Schizochytrium sp. AB‐610 morphological characters in four stages of fed‐batch fermentation under SEM observation illustrated the relation of cell growth and lipid accumulation, which might guide the improvement of fermentation based on routine microscopic observation to enhance DHA accumulation.
3.2. Relation between lipid body morphology and lipid accumulation in fed‐batch fermentation
The differential interference contrast images and fluorescence images via LSCM in Fig. 3 showed the significant intracellular changes of lipid bodies along the whole fed‐batch fermentation. The lipid bodies were reflected as red stains in fluorescence images.
Figure 3.
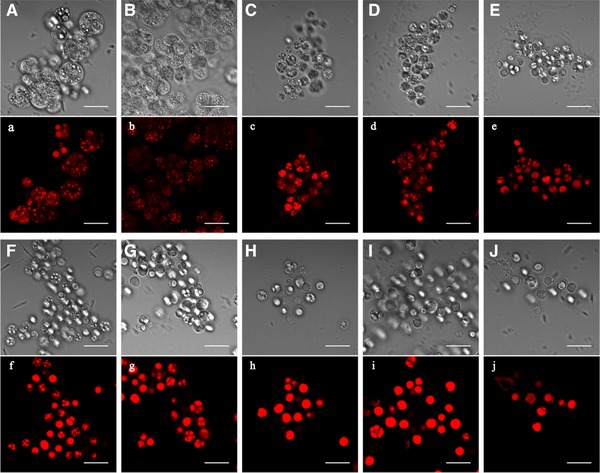
Changes of light microscopic morphology in fed‐batch fermentation by Schizochytrium sp. AB‐610 detected by LSCM after Nile red staining. Cells were cultured for 12 h (A, a), 24 h (B, b), 36 h (C, c), 48 h (D, d), 60 h (E, e), 72 h (F, f), 84 h (G, g), 96 h (H, h), 108 h (I, i), and 120h (J, j). The differential interference contrast images (uppercase letters) and fluorescence images (lowercase letters) of the same cells were shown. Scale bar = 20 μ μm.
During lag stage the lipid bodies in cell were small and dense, spread evenly in the cell (Fig. 3A, a, B and b), and started to increase sizes during balanced growth stage (Fig. 3C, c, D and d). There were almost no cells full of lipid body (FLB) until this stage, and the lipid content accumulated in cells was still relatively low. During lipid accumulation stage, as the lipid content kept increasing, the distributed small lipid bodies started to merge into larger ones (Fig. 3E and e). The amount of zoosporangia gradually reduced, along with FLB cells increased, which had only one brimming whole cell lipid body observed as darker color in microscope (Fig. 3F, f, G and g). The lipid content increased at this stage accompanied with the increase of FLB cell ratio (Fig. 1B), with a linear regression of R 2 = 0.9864. At the end of lipid accumulation stage, FLB cell ratio reached its maximum, and lipid content was highest at the same time. During lipid turnover stage, the proliferating cell rate was lowest. As the lipid became to be consumed, FLB cell ratio under the microscope showed a slightly decrease (Fig. 3I, i, J and j). Therefore, FLB ratio obtained under the microscope well reflected the amount of lipid accumulation during fermentation progress.
The morphological features of the lipid bodies in the synchronous growth of Schizochytrium cells was previously described by 19 via fluorescent and Electron microscopy, that lipid bodies increased both in size and in number as the cells grew. However in our research, the lipid bodies gradually enlarged and merged with each other and finally formed one large lipid body filling the whole cell. It was suggested that the effects of environment on cell proliferation and growth caused the structural differences of lipid body formation, which is worthy of further research.
3.3. FA composition shifts in different morphological stages
In cell growth process, myristic acid (C14:0), palmitic (C16:0), DHA and docosapentaenoic acid (DPA; C22:5) were the predominant cellular FAs, while palmitoleic acid (C16:1), oleic acid (C18:1) acid (C18:0) were detected in low quantities (Table 1). Two FAs biosynthesis pathways were reported exist in Schizochytrium sp.: fatty acid synthase (FAS) pathway and polyketide synthase (PKS) pathway 32. FAS products include short chain saturated FAs (C14:0 and C16:0) and their derivatives through elongations and desaturations such as C16:1, C18:0, C18:1; PKS products are polyunsaturated FAs identified.
Table 1.
FA compositions of Schizochytrium sp. AB‐610 in fed‐batch culture (% total FAs)
| Fermentation stage | Lag stage | Balanced growth | Lipid accumulation stage | Lipid turnover stage | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 |
| C14:0 | 4.24 | 10.59 | 15.35 | 14.54 | 16.05 | 18.35 | 16.49 | 16.95 | 11.86 | 10.00 |
| C16:0 | 17.46 | 22.71 | 24.12 | 22.78 | 22.83 | 22.11 | 23.78 | 22.93 | 21.88 | 21.06 |
| C16:1 | 0.93 | 0.48 | 0.37 | 0.50 | 1.10 | 0.94 | 0.96 | 0.71 | 0.84 | 0.55 |
| C18:0 | 1.05 | 0.58 | 0.43 | 0.39 | 0.40 | 0.39 | 0.36 | 0.40 | 0.33 | 0.34 |
| C18:1 | 1.16 | 0.30 | 0.22 | 0.27 | 0.45 | 0.31 | 0.20 | 0.46 | 0.95 | 0.65 |
| DPA | 11.31 | 13.74 | 12.19 | 12.23 | 12.81 | 12.59 | 12.60 | 11.64 | 14.16 | 14.94 |
| DHA | 48.78 | 46.76 | 42.72 | 40.56 | 38.75 | 36.62 | 36.66 | 38.03 | 42.96 | 42.85 |
| Other minor Fas | 15.07 | 6.84 | 5.60 | 9.73 | 7.61 | 8.69 | 9.95 | 8.88 | 7.02 | 8.74 |
| FAS products | 24.84 | 34.66 | 40.49 | 38.48 | 40.83 | 42.10 | 41.79 | 41.45 | 35.86 | 33.47 |
| PKS products | 62.09 | 60.50 | 54.91 | 52.79 | 49.56 | 51.21 | 53.26 | 53.67 | 57.12 | 57.79 |
At the early period of fermentation progress, PKS products were the predominant and reached to 62.09% of total FAs at 12 h (Table 1). DHA almost reached 50% of total FAs at this time. Cells proliferated at peak speed in this period and exhibited relative small in size. At lipid accumulation stage, when cells started to expand as lipid accumulated inside, however, the percentage of PKS products significantly decreased while FAS products showed an increase. DHA percentage also decreased to 42.03% of total FAs at the end of this stage. During lipid turnover stage, when cells wrinkled as lipid started to be consumed, the percentage of PKS products increased to 57.79%, while the percentage of FAS products fell to 33.47%. DHA percentage also showed a slightly rise at this stage.
Those results suggested that PKS system had disadvantages over FAS system in synthesizing their FA products from the required elements of acetyl‐CoA and NADPH in cytoplasm in lipid accumulation stage, which was considered to be the critical period to regulate the microorganism to biosynthesize DHA. The rise of DHA percentage in lipid turnover stage illustrated that PKS products were more difficult to decompose than FAS products. This is in agreement with 30. The FA composition shifts can be determined by observation of cell morphology.
3.4. Two‐stage pH control based on cell morphology to promote Schizochytrium sp. AB‐610 DHA production
3.4.1. DHA fermentation from Schizochytrium sp. AB‐610 at different pH levels
Figure 4A, B shows the time course of DHA fermentation by Schizochytrium sp. AB‐610 at controlled pH levels (of 4.0, 5.0, 6.0, 7.0 and 8.0) in a 7.5 L fermenter. According to Fig. 4A, Schizochytrium sp. AB‐610 accommodated the pH ranging from 4.0 to 8.0 and grew normally. The cells tended to experience less lag time and grew faster when the pH was controlled closer to neutral. The biomass reached highest of 62.63 g/L DCW at 120 h when the pH was controlled at 7.0. The cells appeared binary division earliest at this pH condition. This result illustrated that neutral pH condition is favorite to Schizochytrium sp. AB‐610 cell growth and proliferation. It was possible that neutral pH condition of fermentation media was close to the pH of inoculated seed culture (6.8), so cells were easier to adapt to the new growth condition and spent less time in lag stage.
Figure 4.
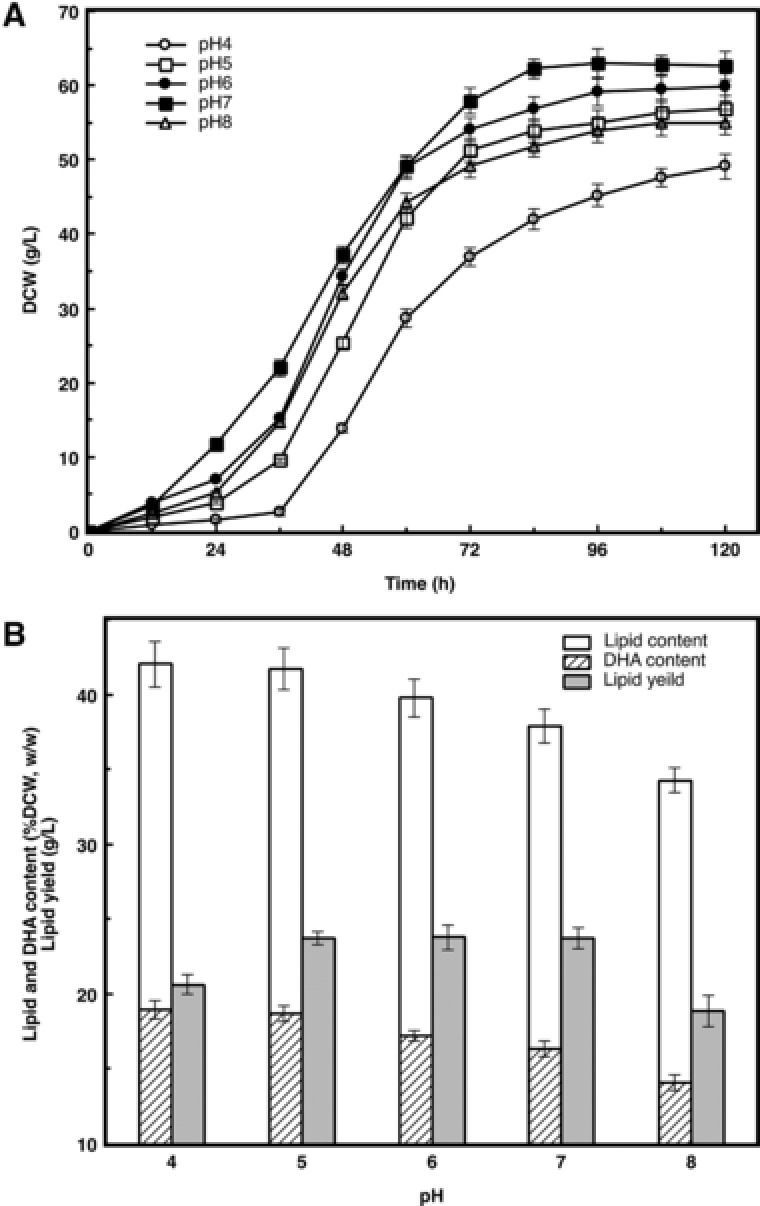
Effect of different pHs on biomass, lipid and DHA productivity of Schizochytriumsp. AB‐610 in fed‐batch fermentation. Seed culture mediums were inoculated into fermentation mediums with different constant pH values ranging from 4.0 to 8.0. DCW (A), Lipid and DHA productivity (B) was measured.
Although the biomass decreased slightly at pH 5.0, the ratios of lipid content to biomass and DHA content to total lipid were reaching to 41.72 and 44.79%, respectively, 10.14 and 3.9% higher compared with that that cultured under neutral pH condition (Fig. 4B). Also, the proliferation period was shortened and the cell expanding period was prolonged. These results seemed consistent with the conclusion of 21 that acidic condition is beneficial for Schizochytrium sp. to accumulate lipid and synthesize DHA. Lipid content of biomass reached highest of 42.01% when pH was controlled as 4.0, but it brought disadvantage as the biomass decreased dramatically, resulting consequently in lower lipid and DHA yield.
Under acidic condition, some fatty acid synthesis related key enzymes may be activated to enhance lipid accumulation and DHA synthesis, but too low acidic condition was suggested to interfere with intracellular enzyme activities and membrane permeability, leading to impact of cell growth and proliferation. Under alkaline condition, some enzyme activity related metal ions, including Mg2+, Fe2+, Ca2+, et al., cannot be dissolved and therefore cannot be utilized, so the lipid and DHA production declined as the pH value rose above 7.
According to the results described above, it is known that cell rapid growth and lipid accumulation appeared in different stages. Beside, pH played an important role in both cell growth and lipid accumulation. In order to obtain the highest DHA yield, biomass, lipid content in biomass and DHA content in total lipid are all crucial. A balance point to regulate pH needs to be found between cell growth time and lipid accumulation time to obtain highest DHA yield.
3.4.2. Implementation of two‐stage pH control strategy on DHA fermentation
Strategy of raising DHA yield was demonstrated in Fig. 5, by regulating pH levels at different fermentation time periods. In each experiment group, pH was set at 7.0 initially and then shifted to 5.0 before, on or after the starting of lipid accumulation stage (48 h) until the end of the fermentation.
Figure 5.
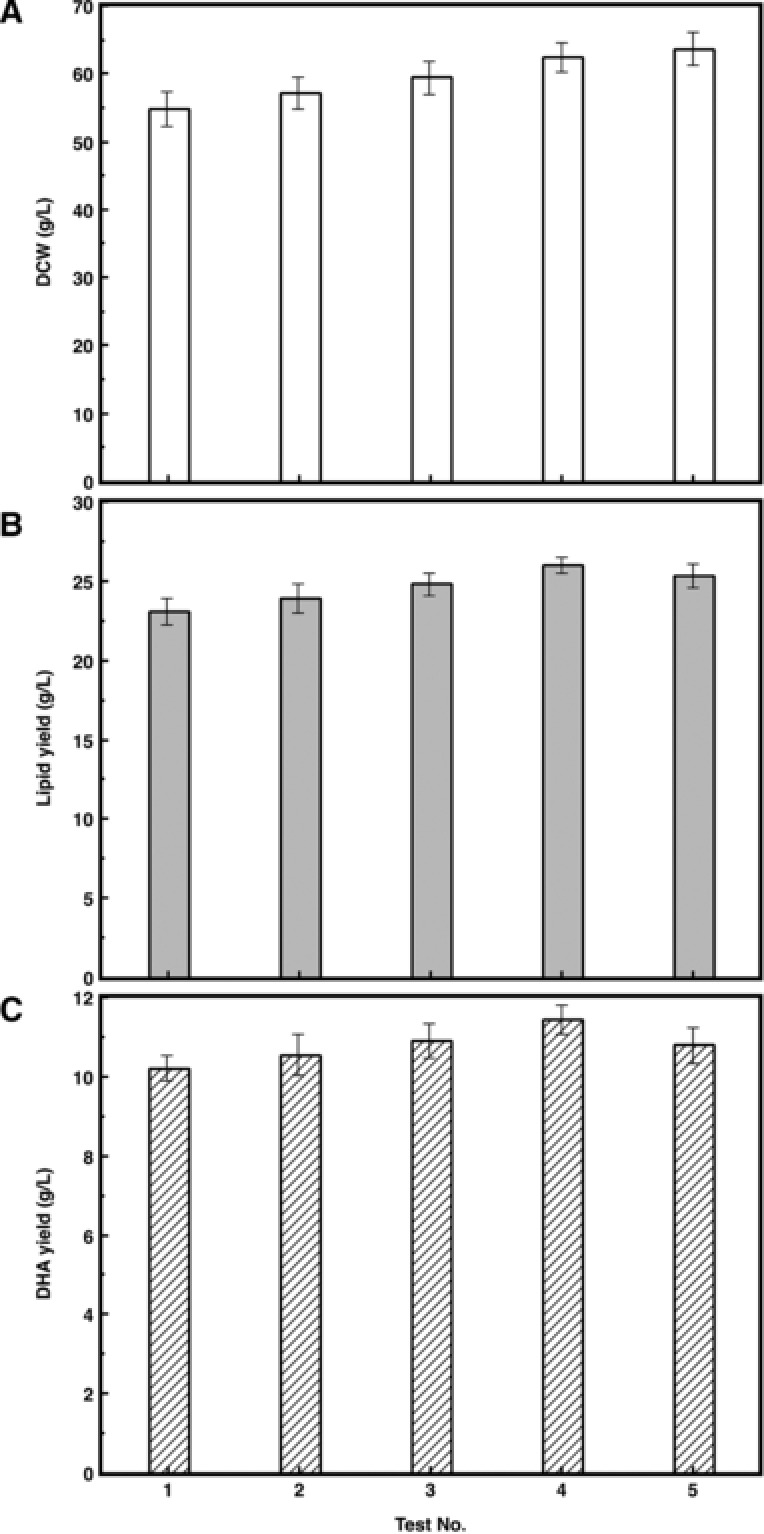
Biomass, lipid, and DHA productivity of Schizochytriumsp. AB‐610 in fed‐batch fermentation under different pH control strategies. Test No. 1–5 represent the pH control strategies that pH was kept at 7 for 24, 36, 48, 60, and 72 h and then shifted to 5.0 and kept to the end of fermentation, respectively. (A): DCW; (B): Lipid yield; (C): DHA yield.
The biomass increased as the neutral pH time extended (Fig. 5A). The lipid and DHA yield also rose with the increase of biomass, and reached the highest in test No. 4 (Fig. 5B, C), in which pH shifted from 7.0 to 5.0 at 60 h, 12h after the end of balanced growth stage, when expanding of cell size and the merge of lipid bodies were observed. Extending the neutral pH culture time increased the cell proliferation time, while the lipid accumulation rate was still relatively high. The lipid and DHA yields decreased when the neutral pH time was longer than 60 h, even though the biomass still showed a rising trend. This is because the maintaining time of optimal lipid accumulation pH was shortened too much and there was not enough time for the cell to synthesize lipid at the highest speed, as the cell activity started to decrease at the later stage of the fermentation period.
Fermentation process under optimized pH control strategy was compared with that without pH control (Fig. 6). Lag stage was shortened when the initial pH was controlled at 7, as it was easier for cells to adapt themselves to neutral condition. Lipid yield under pH 7 was also a little higher, contributing to the higher biomass. When the pH shifted to 5, the lipid accumulation speed showed a significant increase compared with the fermentation process without pH control. Under this pH control strategy, the final lipid and DHA yields reached 26.00 and 11.44 g/L, respectively, 19.0 and 22.2% higher than that without pH control strategy (21.85 and 9.36 g/L).
Figure 6.
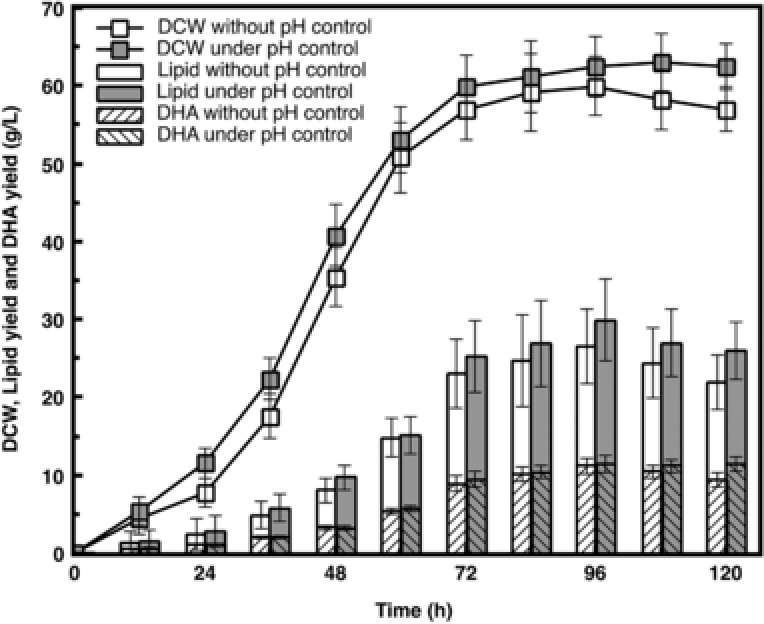
Comparision of Schizochytrium sp. AB‐610 fermentation with and without pH control strategy. DCW, lipid yield and DHA yield under two different fermentation conditions were compared.
4. Concluding remarks
Four stages of DHA fermentation from Schizochytrium sp. AB‐610 were clarified based on the morphologic observation and the test of pH changes. The relations of cell morphological changes, lipid accumulation, and FA composition shifts were also clarified. Furthermore, A two‐stage pH control strategy was simply set by keeping pH 7.0 in the first two stages and the first 12 h of the third stage, then shifted to 5.0 in the rest period, in order to readjust Schizochytrium sp. metabolism and therefore to enhance the yields of lipid and DHA. The above practice provides references to the other useful bioproducts as well as DHA from Schizochytrium sp.
Practical application
Schizochytrium sp. is a recognized species for high yield DHA fermentation. During Schizochytrium sp. AB‐610 fermentation process, it showed DHA production varied with changes of the strain morphology and the pH values. It seemed reasonable to increase DHA production of Schizochytrium sp. AB‐610 by optimizing the relationships of these factors. A simple strategy of two‐stage pH control on fermentation was set to improve DHA production significantly.
The authors have declared no conflict of interest.
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program, Grant No.2014AA021702); the Program of the Innovation Plan of Jiangsu Province (KYLX_1148); the Fundamental Research Funds for the Central Universities (JUSRP51402A); the program of the Key Laboratory of Industrial Biotechnology, Ministry of Education, China (No. KLIB‐KF201601), and the 111 Project (No. 111‐2‐06).
Contributor Information
Hailin Yang, Email: 19891996@sina.com.
Wu Wang, Email: bioprocessor@aliyun.com, Email: wangwujnu@aliyun.com.
5 References
- 1. Ratledge, C. , Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815. [DOI] [PubMed] [Google Scholar]
- 2. Honda, D. , Yokochi, T. , Nakahara, T. , Raghukumar, S. et al., Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of 18S ribosomal RNA gene. J. Eukaryot. Microbiol. 1999, 46, 637–647. [DOI] [PubMed] [Google Scholar]
- 3. Darley, W. M. , Porter, D. , Fuller, M. S. , Cell wall composition and synthesis via Golgi‐directed scale formation in the marine eucaryote, Schizochytrium aggregatum, with a note on Thraustochytrium sp. Arch. Mikrobiol. 1973, 90, 89–106. [DOI] [PubMed] [Google Scholar]
- 4. Jiang, Y. , Fan, K. W. , Wong, R. D. Y. , Chen, F. , Fatty acid composition and squalene content of the marine microalga Schizochytrium mangrovei. J. Agric. Food Chem. 2004, 52, 1196–1200. [DOI] [PubMed] [Google Scholar]
- 5. Barclay, W. , Weaver, C. , Metz, J. , Development of a Docosahexaenoic acid production technology using Schizochytrium: a historical perspective, in: Cohen Z. and Ratledge C., (eds.), Single Cell Oils, AOCS Press, Champaign, Illinois: 2005, pp. 36–52. [Google Scholar]
- 6. Yang, H. L. , Lu, C. K. , Chen, S. F. , Chen, Y. M. et al., Isolation and characterization of Taiwanese heterotrophic microalgae: screening of strains for docosahexaenoic acid (DHA) production. Mar. Biotechnol. 2010, 12, 173–185. [DOI] [PubMed] [Google Scholar]
- 7. Fu, J. , Chen, T. , Lu, H. , Lin, Y. et al., Enhancement of docosahexaenoic acid production by low‐energy ion implantation coupled with screening method based on Sudan black B staining in Schizochytrium sp. Bioresour. Technol. 2016, 221, 405–411. [DOI] [PubMed] [Google Scholar]
- 8. Unagal, P. , Assantachai, C. , Phadungruengluij, S. , Suphantharika, M. et al., Coconut water as a medium additive for the production of docosahexaenoic acid (C22 : 6 n3) by Schizochytrium mangrovei Sk‐02. Bioresour. Technol. 2007, 98, 281–287. [DOI] [PubMed] [Google Scholar]
- 9. Liang, Y. N. , Sarkany, N. , Cui, Y. , Yesuf, J. et al., Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour. Technol. 2010, 101, 3623–3627. [DOI] [PubMed] [Google Scholar]
- 10. Ethier, S. , Woisard, K. , Vaughan, D. , Wen, Z. Y. , Continuous culture of the microalgae Schizochytrium limacinum on biodiesel‐derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011, 102, 88–93. [DOI] [PubMed] [Google Scholar]
- 11. Ganuza, E. , Anderson, A. J. , Ratledge, C. , High‐cell‐density cultivation of Schizochytrium sp in an ammonium/pH‐auxostat fed‐batch system. Biotechnol. Lett. 2008, 30, 1559–1564. [DOI] [PubMed] [Google Scholar]
- 12. Ren, L. J. , Huang, H. , Xiao, A. H. , Lian, M. et al., Enhanced docosahexaenoic acid production by reinforcing acetyl‐CoA and NADPH supply in Schizochytrium sp. HX‐308. Bioprocess Biosyst. Eng. 2009, 32, 837–843. [DOI] [PubMed] [Google Scholar]
- 13. Taoka, Y. , Nagano, N. , Okita, Y. , Izumida, H. et al., Influences of culture temperature on the growth, lipid content and fatty acid composition of Aurantiochytrium sp strain mh0186. Mar. Biotechnol. (NY). 2009, 11, 368–374. [DOI] [PubMed] [Google Scholar]
- 14. Song, X. J. , Zhang, X. C. , Kuang, C. H. , Zhu, L. Y. et al., Optimization of fermentation parameters for the biomass and DHA production of Schizochytrium limacinum OUC88 using response surface methodology. Process Biochem. 2007, 42, 1391–1397. [Google Scholar]
- 15. Huang, T. Y. , Lu, W. C. , Chu, I. M. , A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour. Technol. 2012, 123, 8–14. [DOI] [PubMed] [Google Scholar]
- 16. Ganuza, E. , Izquierdo, M. S. , Lipid accumulation in Schizochytrium G13/2S produced in continuous culture. Appl. Microbiol. Biotechnol. 2007, 76, 985–990. [DOI] [PubMed] [Google Scholar]
- 17. Qu, L. , Ji, X. J. , Ren, L. J. , Nie, Z. K. et al., Enhancement of docosahexaenoic acid production by Schizochytrium sp. using a two‐stage oxygen supply control strategy based on oxygen transfer coefficient. Lett. Appl. Microbiol. 2011, 52, 22–27. [DOI] [PubMed] [Google Scholar]
- 18. Ashford, A. , Barclay, W. R. , Weaver, C. A. , Giddings, T. H. et al., Electron microscopy may reveal structure of docosahexaenoic acid‐rich oil within Schizochytrium sp. Lipids. 2000, 35, 1377–1386. [DOI] [PubMed] [Google Scholar]
- 19. Morita, E. , Kumon, Y. , Nakahara, T. , Kagiwada, S. et al., Docosahexaenoic acid production and lipid‐body formation in Schizochytrium limacinum SR21. Mar. Biotechnol. (NY). 2006, 8, 319–27. [DOI] [PubMed] [Google Scholar]
- 20. Li, X. Y. , Lin, Y. , Chang, M. , Jin, Q. Z. et al., Efficient production of arachidonic acid by Mortierella alpina through integrating fed‐batch culture with a two‐stage pH control strategy. Bioresour. Technol. 2015, 181, 275–282. [DOI] [PubMed] [Google Scholar]
- 21. Nakahara, T. , Yokochi, T. , Higashihara, T. , Tanaka, S. et al., Production of docosahexaenoic and docosapentaenoic acids by Schizochytrium sp. isolated from Yap islands. J. Am. Oil Chem. Soc. 1996, 73, 1421–1426. [Google Scholar]
- 22. Kahar, P. , Iwata, T. , Hiraki, J. , Park, E. Y. et al., Enhancement of epsilon‐polylysine production by Streptomyces albulus strain 410 using pH control. J. Biosci. Bioeng. 2001, 91, 190–194. [DOI] [PubMed] [Google Scholar]
- 23. Hu, Z. C. , Zheng, Y. G. , Wang, Z. , Shen, Y. C. , pH control strategy in astaxanthin fermentation bioprocess by Xanthophyllomyces dendrorhous. Enzyme Microb. Technol. 2006, 39, 586–590. [Google Scholar]
- 24. Ren, L. J. , Ji, X. J. , Huang, H. , Qu, L. A. et al., Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp. Appl. Microbiol. Biotechnol. 2010, 87, 1649–1656. [DOI] [PubMed] [Google Scholar]
- 25. Honda, D. , Yokochi, T. , Nakahara, T. , Erata, M. et al., Schizochytrium limacinum sp. nov., a new thraustochytrid from a mangrove area in the west Pacific Ocean. Mycol. Res. 1998, 102, 439–448. [Google Scholar]
- 26. Meesters, P. A. E. P. , Huijberts, G. N. M. , Eggink, G. , High cell density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar]
- 27. Ho, S. H. , Chen, C. Y. , Chang, J. S. , Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW‐N. Bioresour. Technol. 2012, 113, 244–252. [DOI] [PubMed] [Google Scholar]
- 28. Papanikolaou, S. , Sarantou, S. , Komaitis, M. , Aggelis, G. , Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple‐limited media. J. Appl. Microbiol. 2004, 97, 867–875. [DOI] [PubMed] [Google Scholar]
- 29. Ratledge, C. , Wynn, J. P. , The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–51. [DOI] [PubMed] [Google Scholar]
- 30. Chang, G. , Luo, Z. , Gu, S. , Wu, Q. et al., Fatty acid shifts and metabolic activity changes of Schizochytrium sp. S31 cultured on glycerol. Bioresour. Technol. 2013, 142, 255–260. [DOI] [PubMed] [Google Scholar]
- 31. Boulton, C. A. , Ratledge, C. , Cryptococcus terricolus, an oleaginous yeast re‐appraised. Appl. Microbiol. Biotechnol. 1984, 20, 72–76. [Google Scholar]
- 32. Metz, J. G. , Roessler, P. , Facciotti, D. , Levering, C. et al., Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science. 2001, 293, 290–293. [DOI] [PubMed] [Google Scholar]


