Figure 6.
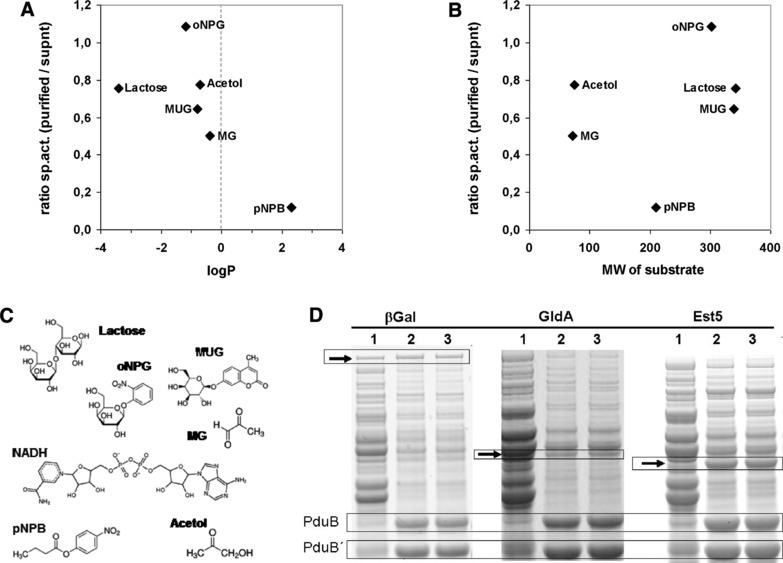
Specific activities of enzymes associated to BMCs correlates with the partition coefficient logP of substrates. The ratio of the specific activities of enzymes associated with BMCs and of the enzymes not associated with BMCs of the same expression experiment was plotted against the logP (A) or the molecular weight (B) of the substrate compounds. LogP describes the distribution of a given compound in an octanol‐water biphasic solution system. Lipophilic compounds have positive logP values and vice versa. In the graphs, a ratio of 1 indicates unrestricted access of substrates to the BMC‐associated enzyme. (C) Molecular structure of the substrate compounds used in this study; abbreviations, see table S6. (D) SDS‐PAGE analyses of the protein samples used for this analysis demonstrate similar amounts of βGal in the soluble and the BMC‐containing fraction, whereas esterase Est5 is strongly enriched in the BMC‐fraction. Arrows indicate relevant protein bands; PduB, PduB´, major shell proteins of the BMC used for comparing protein amounts. Lane 1, supernatant after centrifugation at 20 000 × g, BMCs are largely depleted; lane 2, cleared pellet, i.e. resuspended pellet after the 20 000 × g centrifugation step; lane 3, purified BMCs after affinity purification using magnetic Ni‐beads to deplete unbound enzymes. Protein samples corresponding to lanes 1 and 3 were used for determining the ratio of the specific activities plotted in graph (A) and (B).
