ABSTRACT
Multiple sclerosis (MS) without optic neuritis causes color-vision deficit but the evidence for selective color deficits in parvocellular-Red/Green (PC-RG) and koniocellular-Blue/Yellow (KC-BY) pathways is inconclusive. We investigated selective color-vision deficits at different MS stages. Thirty-one MS and twenty normal participants were tested for achromatic, red-green and blue-yellow sinewave-gratings (0.5 and 2 cycles-per-degree (cpd)) contrast orientation discrimination threshold. Red-green mean threshold at 0.5cpd in established-MS and blue-yellow mean threshold in all MS participants were abnormal. These findings show blue-yellow versus red-green color test is useful in differentiating MS chronicity, which helps to better understand the mechanism of colour-vision involvement in MS.
KEYWORDS: Multiple sclerosis, red/green, blue/yellow, colour vision, psychophysics
Introduction
Multiple sclerosis (MS) is the second common cause of neurological morbidity among young adults in North America.6 For many years, MS, particularly relapsing remitting MS (RRMS), was considered as a typical episodic demyelinating disorder of the central nervous system (CNS). Accumulating axonal damage and neurodegeneration were observed in progressive and RRMS patients. For example, in 1936 Putnam reported a 50% axonal loss in MS lesions of 11 patients. However, neurodegeneration was thought to be secondary to demyelination in RRMS.7 There has been increasing evidence showing that axonal degeneration and neuronal loss is present in early MS, including RRMS, and it is a major contributor in permanent neurological deficit.
The primate visual system has three distinct pathways to distinguish chromatic (i.e. coloured) and achromatic (i.e. grey scale) images. Visual information collection, processing, and transfer start from photoreceptor and is integrated by bipolar and ganglion cells in the retina. The axons from the ganglion cells synapse in the lateral geniculate nuclei, and then reach the primary (V1) and extra-striate (e.g. V4) visual cortices. There are two different types of photoreceptors in the retina (cones and rods), which are sensitive to different light wavelengths.
Three different populations of cone photoreceptors have been identified, i.e. blue, green, and red cones, which are optimally sensitive to short (S) (420 nm), medium (M) (534 nm), and long (L) (564 nm) light wavelengths, respectively. Hence, the three types of cone photoreceptors have specific functional roles in colour vision.8–10 On the other hand, rod photoreceptors are optimally sensitive to medium light wavelength of 498 nm and carry grey scale luminance/contrast visual information.
The parvocellular pathway starts from cone photoreceptors in the retina and projects to P-cell layers of the lateral geniculate nucleus (so called P-pathway). The cone photoreceptors are denser in the fovea of the retina. P-pathway is highly sensitivity to red–green (RG) colour contrast and is responsible for RG colour vision.4,11,12
The magnocellular pathway starts from rod photoreceptors, which are distributed in parafoveal and peripheral retinal areas, and projects to the M-cell layers in the lateral geniculate nucleus (so called M-pathway). This pathway carries mainly achromatic visual information.4,11,13
The third pathway is specialised for distinguishing blue–yellow (BY) colours. This pathway receives its input from bistratified retinal ganglion cells and projects to the koniocellular layers of the lateral geniculate nucleus.4,9,14,15 Unlike RG pathway, BY pathway cells are sparse and are found adjacent to central vision.16,17
Acute optic neuritis (ON) is the presenting symptom in 20% of patients with MS.18 Despite full recovery of visual acuity, patients with ON will suffer from long-lasting loss of spatial, temporal, luminance, and/or chromatic visual function.18,19
Previously, it was thought that MS damages visual pathway merely through involving oligodendrocytes and demyelination of the optic nerve, i.e. clinical ON. Recently, it has been shown that the optic nerve and retina are affected in MS patients without any clinical evidence or history of ON.2–4 Given that there is no myelin and oligodendrocytes in the retina, retinal damage has been hypothesized to be secondary to retrograde trans-synaptic neurodegeneration as a result of damage to optic nerve and chiasmatic/post-chiasmatic visual pathways. Alternatively, retinal and optic nerve damage without a history of ON may indicate a primary ocular neurodegenerative process that represents the extent of cortical neurodegeneration in MS.
It has been shown that ON affects the parvocellular pathway to a greater extent compared with koniocellular or achromatic pathways. The difference in colour vision loss is more apparent with high spatial and high temporal frequency stimuli.8,17 The evidence for selective chromatic loss in demyelinating diseases is still inconclusive. Some studies have shown RG, whereas some have shown BY axis abnormalities.4,5
Most of the previous studies that showed colour vision deficit in demyelinating diseases have used Ishihara pseudoisochromatic plates (Ishihara) or the Farnsworth-Munsell (FM) 100 hue test. Using these tests in a clinical environment is problematic. On one hand, the Ishihara test is designed to assess RG colour vision deficiencies and has limitations in recognizing BY colour vision defects. On the other hand, performing the FM 100 hue test is time consuming and not practical in a clinic environment.2–4,20 The Farnsworth D-15 panel is another colour vision test that was developed as a colour vision grading test for use in vocational guidance.21,22 This test is not able to detect minor abnormalities in colour vision.23
Although colour vision impairment in MS patients without a history of ON has been documented in previous studies, comparison of the colour vision defect in patients at different stages of the disease has not been evaluated.
The aim of this study was to assess spatial chromatic discrimination in the eyes of MS patients those not previously affected by ON, at early and late stages of the MS, using an affordable, fast, simple, and accurate psychophysical computerised colour vision test in order to determine the early involvement of the magnocellular versus parvocellular versus koniocellular pathways in MS patients. Moreover, we aimed to compare the degree of the colour vision abnormality at different stages of the disease, which can be helpful to guide future longitudinal studies to evaluate using colour vision as a possible indicator of diagnosis, progression, and prognosis in MS. There are no established criteria for early versus established MS in the literature, therefore, we defined the RRMS patients as early-MS who were within one year of their diagnosis, and established-MS as RRMS patients who were between 5 and 10 years after diagnosis as per recommendation of one of our co-authors (RV) who is an expert in the MS field and has been practising for more than 25 years.
To our knowledge this is the first chromatic and achromatic psychophysical vision study has investigated early- versus established-MS. Our findings also address the discrepancy in the literature regarding selective colour vision involvement in MS.
Methods
Participants
We tested 16 RRMS patients in the first year of their presentation (6 males and 10 females, between 19 and 59 years of age with a mean age of 33 years) and 15 RRMS patients after the fifth and before the tenth year of diagnosis (two males and 13 females, between 24 and 54 years of age with a mean age of 38 years).
All the participants fulfilled the 2010 McDonald criteria for RRMS and had at least one healthy eye without a history of ON. Participants were recruited through the MS clinic at Health Sciences Centre, and Neuro-ophthalmology clinic in Winnipeg, Manitoba. Data were collected from Jan 2013 to Jan 2015.
From 16 early MS patients, three had CIS and five had a history of ON in one eye. Two of the CIS patients were determined to be high risk in the follow up visits at the MS clinic and showed dissemination in space on MRI and one was low risk without evidence of dissemination in space on brain and spinal cord MRI. From 15 established MS patients, five had a history of ON in one eye.
A Complete neuro-ophthalmologic examination was performed on all the participants, and none of them were diagnosed with any other neuro-ophthalmological or ophthalmological disorders, including cataract, colour blindness (i.e. normal Ishihara test), glaucoma, or changes in visual acuity, which could not be corrected with glasses. Twenty age- and sex-matched controls were randomly selected, between the ages of 19 and 57 with a mean age of 35 years.
Equipment
Chromatic and achromatic orientation discrimination was tested using Psykinamatix v1.4 software (KyberVision, Sendai, Miyagi, Japan) run on a Macbook Pro computer. Stimuli were displayed on a 24 inch, VCR monitor (Dell U2412M, resolution of 1,024 x 768). The monitor was calibrated for chromatic and achromatic luminance using a Spyder4Elite colourimeter (Datacolor, Lawrenceville, New Jersey, USA, https://www.datacolour.com). Several staircase measurements were randomly interleaved to measure contrast threshold as a function of spatial frequency (SF = 0.5 and 2 cpd). In each staircase, the stimulus contrast was reduced after two consecutive correct responses and increased after one wrong response, corresponding to a criterion of 79.1% correct responses. The initial contrast value was randomly selected in the range 90 ± 10. The reduction rate in contrast was 50% before and 12.5% after the first reversal, while the increase rate was always 25%. Each session was terminated after six reversals, and the contrast threshold was computed from the mean of the last five reversals.
Stimuli
The stimuli were horizontal or vertical achromatic (Figure 1a), chromatic red/green (Figure 1b), and chromatic blue/yellow (Figure 1c) sinusoidal Gabors.24
Figure 1.
Horizontal achromatic, vertical red–green and horizontal blue–yellow sinusoidal Gabors presented to participants and controls.
Procedure
The tests were performed in a dark room with the experimenter’s screen off. Each participant was seated 60 cm away from the monitor. The participants without a history of ON were tested monocularly in both eyes with one eye patched at a time, and participants with previous ON were tested only in the unaffected eye. Patients with refractive error (e.g. myopia) used their corrective measures (e.g. glasses or contact lens) during the tests. The Participants’ task was to discriminate the stimulus orientation that was randomly selected by the computer programme in each trial to either vertical or horizontal. The Participants provided their responses using the arrow keys on a keyboard. They were allowed to respond only during the post-stimulus interval. An auditory indicator was emitted at the beginning of each stimulus interval. The overall duration of each stimulus interval was 1 s, and its contrast was temporally modulated by a raised-cosine envelope with a sigma of 0.25 s centred on the temporal window and a flat part of 0.5 s. Auditory feedback was given after each trial. The duration of the inter-trial intervals was 0.5 s.
Statistical analysis
Statistical analyses were performed using Statistical Analysis System (SAS Institute, Cary, North Carolina, USA) software. In order to decrease the eye/inter-eye-correlation we used mixed model effects. ANOVA was used to compare the average contrast thresholds of RG, BY, and achromatic stimulations in two SFs among early MS, established MS and controls.
Results
We measured chromatic (i.e. BY and RG) and achromatic contrast threshold at 0.5 and 2 cpd in 27 eyes in the early MS group, 25 eyes in the established MS group, all without a history of ON, and 40 eyes from 20 age- and sex- matched control group (for overview of the subjects characteristics see Table 1). The mean age was lower in the established MS group compared with the early MS group (32.87 years vs. 37.8 years, respectively). However, this difference in mean age was not statistically different. The mean duration of the MS from diagnosis was 9.15 months in the early MS group and 88.8 months in the established MS group. The achromatic mean contrast thresholds at 0.5 and 2 cpd were 3.47%, 2.41% for early MS, 3.59%, and 1.64% for established MS and 3.04% and 2.57% for controls. p values in all categories of this group were not statistically significant (see Figure 2a and Table 2 for p values).
Table 1.
Demographic characteristics of the patients and controls.
| Early MS | Established MS | Control | |
|---|---|---|---|
| Number of the tested eyes | 27 | 25 | 40 |
| Sex (male/female) | 6/10 | 2/13 | 5/15 |
| Mean Age (years) | 37.8 | 32.87 | 34.85 |
| Mean duration (months) | 9.15 | 88.8 | __ |
| Previous optic neuritis in the fellow eye | 5 | 5 | __ |
| Clinically isolated syndrome | 3 | 0 | __ |
Figure 2.
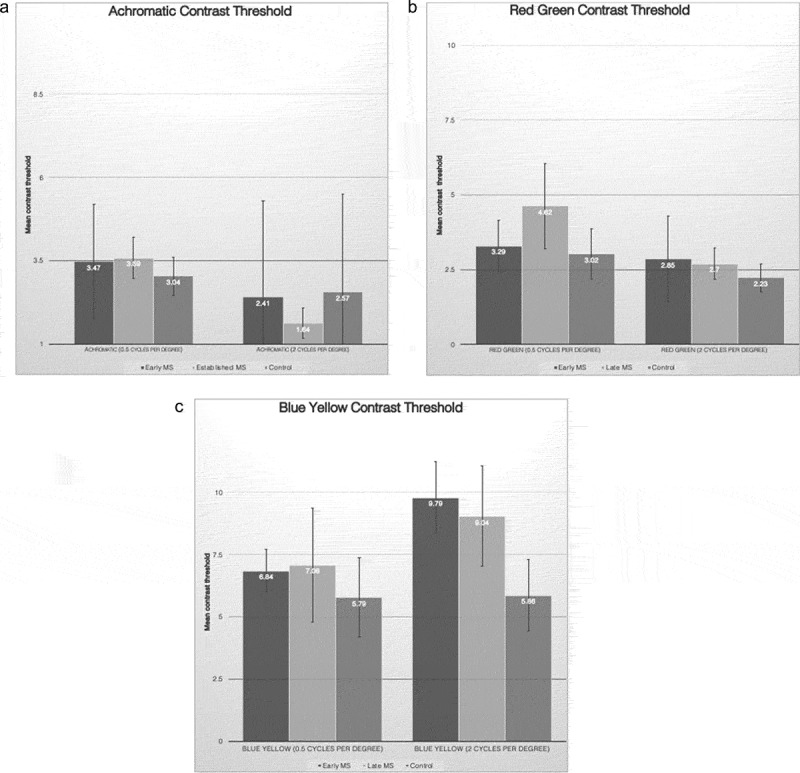
(a) Mean contrast threshold for achromatic stimuli at 0.5 and 2 cycles per degree SF. The difference in the mean contrast threshold of early MS, established MS and controls was not statistically significant. Error bars represent ± 0.5 standard deviation. (b) Mean contrast threshold for red–green stimuli at 0.5 and 2 cycles per degree SF. The mean contrast threshold was significantly elevated in established MS at SF of 0.5 compared with controls. Error bars represent ± 0.5 standard deviation. (c) Mean contrast threshold for blue–yellow stimuli at 0.5 and 2 cycles per degree SF. The mean contrast threshold at 2 cycles per degree was significantly higher in early and established MS versus controls. Error bars represent ± 0.5 standard deviation.
Table 2.
Mean threshold for achromatic, red–green and yellow contrast sensitivity in patients and controls with p values compared with control.
| Stimulus (SF) | Early MS Mean (SD) | p value* | Established MS Mean (SD) | p value* | Control Mean (SD) |
|---|---|---|---|---|---|
| Achromatic (0.5) | 3.47 (1.73) | 0.31 | 3.59 (1.22) | 0.14 | 3.04 (1.13) |
| Achromatic (2) | 2.41 (2.88) | 0.2 | 1.64 (0.91) | 0.46 | 2.57 (5.86) |
| Red green (0.5) | 3.29 (1.87) | 0.52 | 4.62 (2.87) | 0.02 | 3.02 (1.68) |
| Red green (2) | 2.85 (1.89) | 0.17 | 2.7 (1.05) | 0.11 | 2.23 (0.94) |
| Blue yellow (0.5) | 6.84 (6.62) | 0.41 | 7.08 (4.58) | 0.25 | 5.79 (3.18) |
| Blue yellow (2) | 9.79 (7.8) | 0.05 | 9.04 (4.03) | 0.006 | 5.86 (2.86) |
*Compared to control.
Chromatic RG mean contrast thresholds at 0.5 and 2 cpd were 3.29% and 2.85% for early MS, 4.62% and 2.7% for established MS, and 3.02% and 2.23% for controls. The mean contrast threshold was significantly elevated in established MS at an SF of 0.5 compared with controls (p value: 0.02) (see Figure 2b).
Chromatic BY mean contrast thresholds at 0.5 and 2 cpd were 6.84% and 9.79% for early MS, 7.08% and 9.04% for established MS, and 5.79% and 5.86% for controls. The mean contrast threshold at 2 cpd was significantly higher in early and established MS versus controls (p = .05 for early MS vs. controls and p = .006 for established MS vs. controls). There was a trend of a similar pattern of higher contrast threshold in early and established MS compared with that in the control group at 0.5 cpd, though the difference was not statistically significant (see Figure 2c and Table 2).
Discussion
MS is a devastating neurological disease, a major cause of morbidity and loss of quality of life and workforce. The early diagnosis of MS is important as early treatment affects the prognosis of the disease.
Our data show statistically significant loss of BY colour orientation discrimination at 2 cpd in both early and established MS groups without a history of ON compared with that in controls. At 0.5 cpd, there was a similar trend that did not reach statistical significance. Short wavelength (S) cones are associated specifically with the BY (KC) pathway. We also found that RG colour vision was statistically abnormal at 0 cpd (but not at 2 cpd) and for established MS. Medium (M) and long (L) wavelength cones are associated with the RG pathway.
This interesting discrepancy between colour vision sensitivity at high and low special frequencies can be caused by other image attributes of a colour test, such as spatial resolution, which plays a significant role in the sensitivity of the test in showing subtle colour vision losses in MS. Alternatively, this finding can be caused by the number of participants in our study despite the fact that the number of patients in our study is comparable to similar studies in this topic.4
Previous clinical studies in colour vision in MS have mainly used the conventional clinical colour tests including Ishihara colour plates, Hardy-Rand-Rittler pesudoisochromatic plates (HRR) and FM 100 hue. Although these tests have been shown to be useful in some clinical and research circumstances, they inherently have some scientific or practical shortcomings that make their use in early diagnosis of MS suboptimal. For example, the Ishihara colour test has been designed for gross RG colour vision abnormality and are not sensitive to BY colour vision deficits. As discussed in the results section, a major finding in our study was the abnormality in BY vision in early and established MS at 2 cpd, which explains our finding that the Ishihara test was normal in all of our participants and was unable to show any colour vision deficit in our MS patient population. Moreover, the conventional colour vision tests are broadband in spatial resolution, hence despite the fact that our established MS patients were deficient in RG colour vision at 0.5 cpd, the Ishihara colour test was unable to show this deficit.
HRR and Ishihara tests have been extensively used in the past to assess colour vision; however, lack of accurate scoring criteria for classifying neurological defects on the basis of test performance and the number of errors on these tests provides little information on the type or extent of a colour vision defect. The FM 100 hue test is a sensitive test for hue discrimination for RG and BY but it is not sensitive to spatial resolution. Also, it is a very time consuming test so it is not a proper clinical test and it needs manual dexterity, which can be challenging for some MS patients.25 A recent study by Yuksel et al. failed to show statistically a significant colour vision deficit in MS patients without ON despite significant changes in visual evoked potential and optical coherence tomography (OCT) measurements.26 This result might be due to low sensitivity of the FM 100 hue test in detecting subtle colour vision changes or a relatively small sample size. The psychophysical colour test is much less time consuming compared with traditional methods, such as the FM 100 hue test, and is easier to apply.
Two groups have assessed colour vision in non-ON eyes of patients with MS in the past.2,3 Both studies have shown significant deficits in RG vision. These two studies did not separate their participants according to the duration of the disease, which might explain the discrepancy between their and our results.
We showed that 2 cpd BY colour discrimination was abnormal in the early stages of MS in our patient population whereas the RG axis was not affected. The BY pathway has been reported in most mammals and phylogenetically is an ancient pathway.27 Therefore, it is a robust visual pathway and early involvement of the high frequency BY axis cannot be explained by merely the sensitivity of this pathway. In order to perceive high SFs, the visual detector i.e. photoreceptors in the retina, must have a high density so that they can detect high spatial resolution. However, it has been shown that S cones are relatively sparse in the retina and therefore the early involvement of the BY axis in MS might be due to the sparsity of the S cones. This phenomenon has been shown in the studies in Parkinson’s disease (PD), where the longer distance between S cones may be the predominant reason for BY vision loss. The interplexiform and amacrine cells are affected in PD as well.16,28–31 These cells are involved in contrast sensitivity and influence the centre/surround inhibition across considerable distances as well. Previous studies that have evaluated changes in the pattern of retinal thickness in patients with MS showed that the largest differences between the eyes of MS patients and controls were found in the peripapillary retinal nerve fibre layer (RNFL) and macular ganglion cell inner plexiform layer (GCIPL). This is the area with the highest concentration of BY receptors, which is compatible with the results in our study.25
Our results also showed that achromatic vision was not different in MS patients versus controls, which indicates that the conventional contrast sensitivity assessment that are routinely used in the ophthalmology and neurology clinics, e.g. Pelli Robson contrast sensitivity chart test, is not sensitive to detect early visual changes in MS patients.
Our results suggest that detailed and accurate testing of BY and RG pathways might have diagnostic value in patients at different stages of MS where comparing the function of BY and RG pathways might be an indication of progression of the disease. The nerve fibres in the retina are not myelinated so different effects of MS on BY and RG colour vision might be secondary to demyelination in the optic nerve itself or beyond (e.g. chiasm, lateral geniculate nucleus, optic radiation, or cortex). However, the RG and BY pathways are only distinctly separated at the level of the photoreceptors and the retina. Hence, damage to the visual pathway at the retinal level can best explain our result. Such damage can happen either from a retrograde lesion of the retina after demyelination of the optic nerve, which was not the case in our MS patients given that none of them had a history of ON. Therefore, a more reasonable explanation for different BY versus RG colour vision is a neurodegenerative process at the retinal level that may indicate the overall cortical neurodegeneration. The findings of retinal structural studies where they showed RNFL and GCIPL loss in MS patients without ON supports the latter hypothesis.
The current interest in the development of therapies for MS requires sensitive and accurate markers of the demyelination as well as neurodegeneration, and their responses to the therapies. Psychophysical colour vision tests can potentially become more accessible and user friendly, like automated visual field tests, and they can become useful tests for early diagnosis and response to treatment in MS, particularly the neurodegenerative process, in patients with more complicated history and less definitive MRI findings.
We acknowledge that although we performed Ishihara colour tests in all participants, we did not compare the psychophysical tests with other conventional colour tests such as the FM 100 hue and HRR tests in our study. Further investigation in colour vision function along with the structural studies of the retina, i.e. OCT and MRI correlation and comparison of psychophysical colour vision testing with older quantitative colour vison testing are needed to better understand colour dysfunction in MS. To the best of our knowledge, we are the first group who have evaluated quantitative colour vision at two different stage of MS using a quantitative psychophysical test.
Funding Statement
This work was supported by the Manitoba Medical Services Foundation [8-2013-10].
Acknowledgements
This study was supported by funding from Manitoba Medical Service Foundation (MMSF) to NA. We are grateful to Dr. M. Wallace and Ms. P. Singh for their help in data collection and to Dr. W. Beaudot for providing and supporting with Psykinematix computer programme. We are also thankful to HSC research foundation for providing space for our research.
References
- 1.Harrison AC, Becker WJ, Stell WK.. Colour vision abnormalities in multiple sclerosis. Can J Neurol Sci. 1987;14:279–285. [DOI] [PubMed] [Google Scholar]
- 2.Moura AL, Teixeira RA, Oiwa NN, et al. Chromatic discrimination losses in multiple sclerosis patients with and without optic neuritis using the Cambridge colour test. Vis Neurosci. 2008;25:463–468. doi: 10.1017/S0952523808080437. [DOI] [PubMed] [Google Scholar]
- 3.Gundogan FC, Tas A, Altun S, Oz O, Erdem U, Sobaci G. Colour vision versus pattern visual evoked potentials in the assessment of subclinical optic pathway involvement in multiple sclerosis. Indian J Ophthalmol. 2013;61:100–103. doi: 10.4103/0301-4738.99842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hashmi AM, Kramer DJ, Mullen KT. Human vision with a lesion of the parvocellular pathway: an optic neuritis model for selective contrast sensitivity deficits with severe loss of midget ganglion cell function. Exp Brain Res. 2011;215:293–305. doi: 10.1007/s00221-011-2896-4. [DOI] [PubMed] [Google Scholar]
- 5.Travis D, Thompson P. Spatiotemporal contrast sensitivity and colour vision in multiple sclerosis. Brain. 1989;112(Pt 2):283–303. [DOI] [PubMed] [Google Scholar]
- 6.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 7.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 8.Calkins DJ, Sterling P. Evidence that circuits for spatial and color vision segregate at the first retinal synapse. Neuron. 1999;24:313–321. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee S, Callaway EM. Parallel colour-opponent pathways to primary visual cortex. Nature. 2003;426:668–671. doi: 10.1038/nature02167. [DOI] [PubMed] [Google Scholar]
- 10.Foxe JJ, Strugstad EC, Sehatpour P, et al. Parvocellular and magnocellular contributions to the initial generators of the visual evoked potential: high-density electrical mapping of the “C1” component. Brain Topogr. 2008;21:11–21. doi: 10.1007/s10548-008-0063-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee BB, Pokorny J, Smith VC, Martin PR, Valbergt A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J Opt Soc Am A. 1990;7:2223–2236. [DOI] [PubMed] [Google Scholar]
- 12.Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurons in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin PR, White AJ, Goodchild AK, Wilder HD, Sefton AE. Evidence that blue-on cells are part of the third geniculocortical pathway in primates. Eur J Neurosci. 1997;9:1536–1541. [DOI] [PubMed] [Google Scholar]
- 15.Dacey DM, Packer OS. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Curr Opin Neurobiol. 2003;13:421–427. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen-Legros J. Functional neuroarchitecture of the retina: hypothesis on the dysfunction of retinal dopaminergic circuitry in Parkinson’s disease. Surg Radiol Anat. 1988;10:137–144. [DOI] [PubMed] [Google Scholar]
- 17.Dacey CM, Nelson WM, Aikman KG. Prevalency rate and personality comparisons of bulimic and normal adolescents. Child Psychiatry Hum Dev. 1990;20:243–251. [DOI] [PubMed] [Google Scholar]
- 18.Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326:581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- 19.Cao D, Zele AJ, Pokorny J, et al. Functional loss in the magnocellular and parvocellular pathways in patients with optic neuritis. Invest Ophthalmol Vis Sci. 2011;52:8900–8907. doi: 10.1167/iovs.11-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pokorny J, Collins B, Howett G, et al. Procedures for Testing Color Vision: Report of Working Group 41 Committee on Vision. Washington (DC): National Academies Press; 1981. [PubMed] [Google Scholar]
- 21.Birch J. Failure of concordance of the Farnsworth D15 test and the Nagel anomaloscope matching range in anomalous trichromatism. Vis Neurosci. 2008;25:451–453. doi: 10.1017/S0952523808080231. [DOI] [PubMed] [Google Scholar]
- 22.Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- 23.Farnsworth D. The Farnsworth Dichtotomous Test for Colour Blindness Panel D15 Test Manual. NewYork (NY): Psychological Corporation; 1947. [Google Scholar]
- 24.Foley A, Srinivasa Varadharajan A, Chin C, et al. Detection of Gabor patterns of different sizes, shapes, phases and eccentricities. Vision Res. 2007;47:85–107. doi: 10.1016/j.visres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petzold A, Bacler LJ, Calabreci PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16:797–812. doi: 10.1016/S1474-4422(17)30122-9. [DOI] [PubMed] [Google Scholar]
- 26.Yuskel B, Ducel B, Koctekin B, et al. Color vision testing versus pattern visual evoked potentials and optical coherence tomography parameters in subclinical optic nerve involvement in multiple sclerosis. J of Clinic Neurosci. In press. [DOI] [PubMed] [Google Scholar]
- 27.Szmajda BA, Buzas P, FitzGibbon T, Martin PR. Geniculocortical relay of blue-off signals in the primate visual system. Proc Natl Acad Sci USA. 2006;103:19512–19517. doi: 10.1073/pnas.0606970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roodhooft JM. Ocular problems in early stages of multiple sclerosis. Bull Soc Belge Ophtalmol. 2009;313:65–68. [PubMed] [Google Scholar]
- 29.Bowmaker JK, Astell S, Hunt DM, Mollon JD. Photosensitive and photostable pigments in the retinae of old world monkeys. J Exp Biol. 1991;156:1–19. [DOI] [PubMed] [Google Scholar]
- 30.Ehinger B. Connexions between retinal neurons with identified neurotransmitters. Vision Res. 1983;23:1281–1291. [DOI] [PubMed] [Google Scholar]
- 31.Haug BA, Kolle RU, Trenkwalder C, Woelter WH, Paulus W. Predominant affection of the blue cone pathway in Parkinson’s disease. Brain. 1995;118 (Pt 3):771–778. [DOI] [PubMed] [Google Scholar]


