Figure 1. Dendritic cells (DCs) pulsed with TAT–LACK are superior in priming and restimulation of antigen-specific CD8+ T cells when compared with LACK DCs.
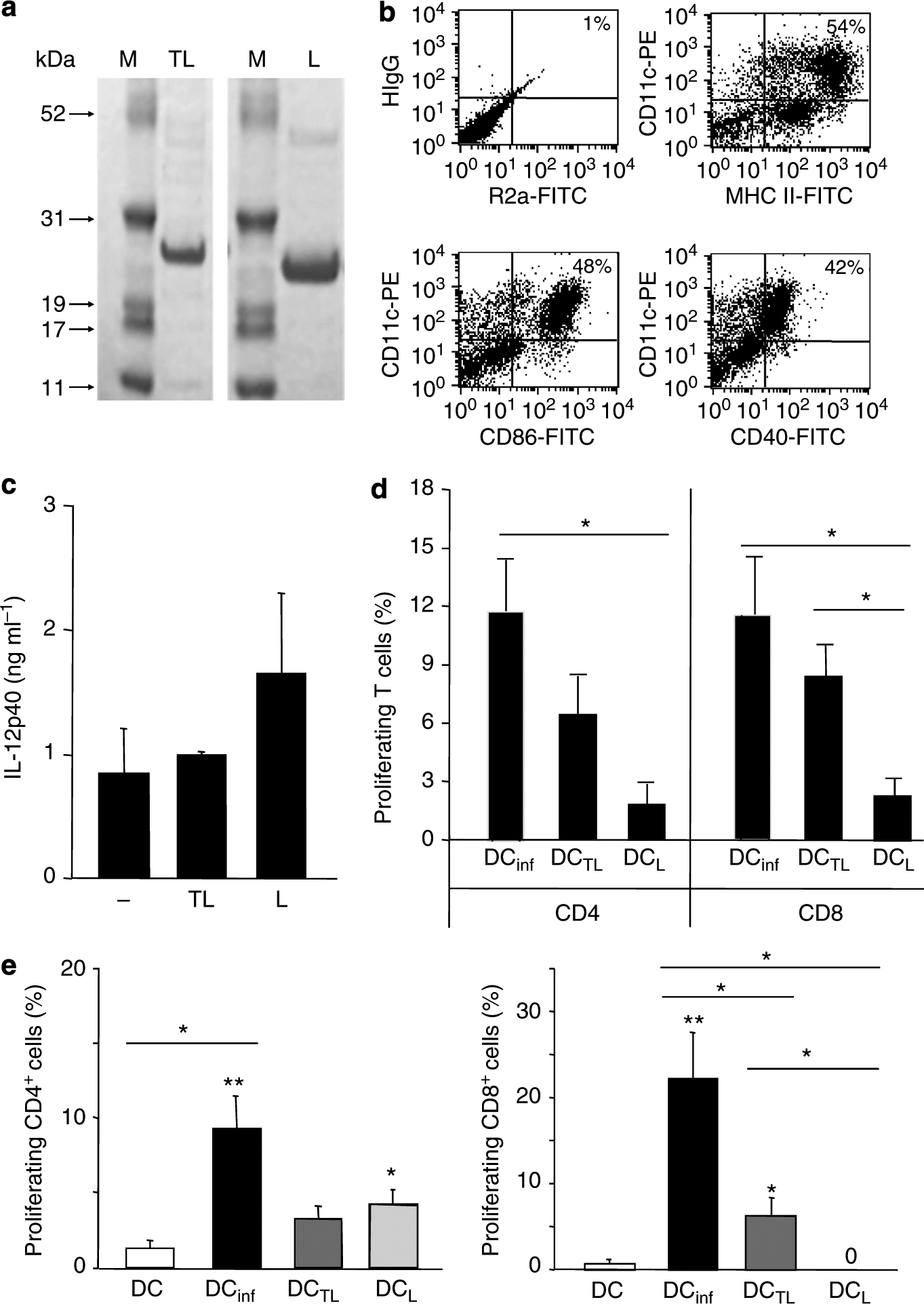
(a) Coomassie blue-stained 4–12% SDS-PAGE gel in which purified TAT–LACK and LACK were resolved. (b) Bone marrow-derived, immature DCs were generated with rIL-4 and rGM-CSF (10 ng ml−1) and harvested as immature DCs on day 6. Expression of activation markers was analyzed by flow cytometry using monoclonal antibodies against CD40, CD86, and MHC class II as well as rat anti-mouse IgG2a (R2a) and hamster anti-mouse IgG1 (HIgG) as isotype controls. One representative FACS staining is shown. (c) DCs were pulsed for 18 hours with TAT–LACK (TL) or LACK (L), or left untreated. IL-12p40 release was determined by ELISA (mean ± SEM, n⩾3). (d) TAT–LACK (DCTL)-treated and LACK (DCL)-treated DCs were obtained or DCs were infected with L. major amastigotes (DCinf, ratio 1:5). T cells were enriched from 6-week L. major-infected C57BL/6 mice using anti-Thy1.2-coated microbeads, labeled with CFSE, and restimulated with irradiated, antigen-pulsed or infected DCs (1:10 ratio of DC/T cells, 1 × 106 per 200 μl). T-cell proliferation was determined after 5 days using flow cytometry. (e) C57BL/6 mice were vaccinated with 2 × 105 untreated DCs, DCinf, DCTL, or DCL. After 1 week, lymph node (LN) cells were isolated, labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), and antigen-specific proliferation was determined by FACS after 5 days of subculture in the presence of soluble Leishmania antigen (SLA). T cells were pregated using staining for CD4 or CD8. (d, e) For each mouse, the relative number of Leishmania-reactive cells (in percentage of total CD4+ or CD8+ T cells) was calculated when compared with untreated control cultures (mean ± SEM, n⩾3, *P⩽0.05, and **P⩽0.005 when compared with DC alone; *above lines indicate differences between stimulation groups).
