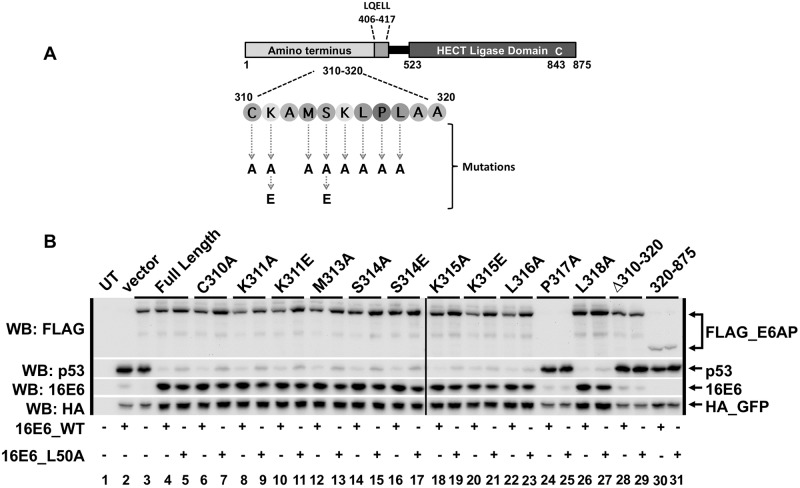
Fig 5. No single amino acid point mutation within E6AP region 310–320 prevents 16E6_WT from initiating degradation of p53.
(A) Schematic of full length E6AP protein. The amino acids located between 310 and 320 (inclusive) are depicted, as is the location of the HECT ligase domain and the active cysteine residue (C843), responsible for ubiquitination of p53. (B) Full length E6AP containing single amino acid point mutations within the 310–320 region still degrade p53 in the presence of 16E6_WT. Plasmids encoding the indicated FLAG_E6AP (1.25 ug), human p53 (0.5 ug), HA_GFP (0.01 ug), and either 16E6_WT or 16E6_L50A (2 ug) were co-transfected into E6AP-null 8B9 cells. Δ310–320 is full length E6AP_WT deleted of residues 310–320 (inclusive). Amino terminal E6AP truncation 320–875 was used as a negative control for p53 degradation in the presence of either 16E6_WT or 16E6_L50A. Vertical black line indicates samples were run on two different western blots. UT = untransfected.