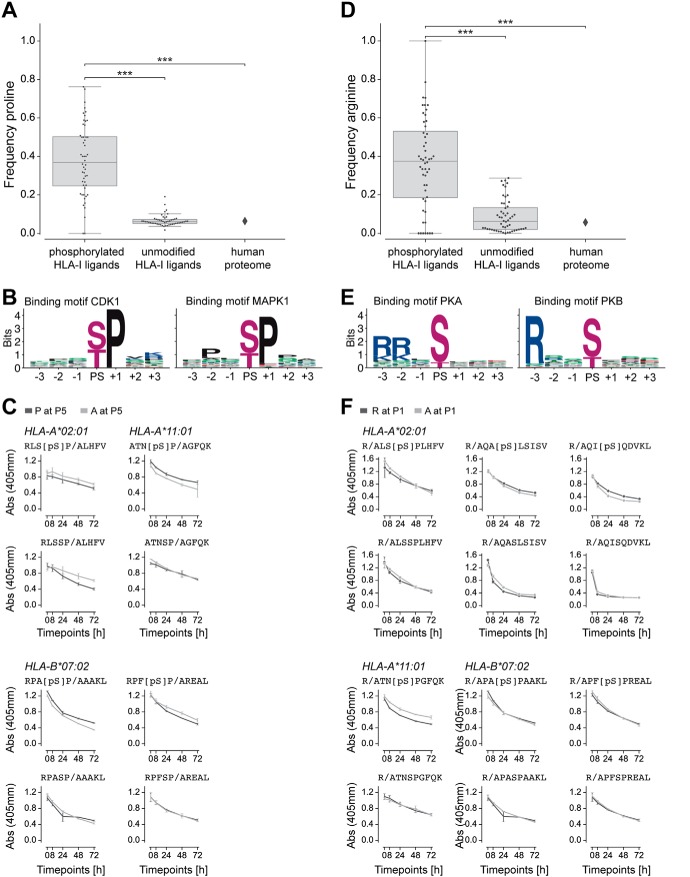
Fig. 4.
Proline and arginine enrichment in phosphorylated HLA-I ligands. A, Frequency of proline next to phosphorylated residues in phosphorylated HLA-I ligands, proline at non-anchor positions in unmodified HLA-I ligands, and proline frequency in the human proteome. B, Kinase binding motifs for kinases CDK1 and MAPK1, three positions up- and downstream of the phosphosite (PS). C, Dissociation of peptides with proline or alanine next to phosphorylated serine (top) and next to unmodified serine in unmodified versions of the peptides (bottom). D, Frequency of arginine at P1 in phosphorylated HLA-I ligands, in unmodified HLA-I ligands, and in the human proteome. E, Kinase binding motifs for kinases PKA and PKB, three positions up- and downstream of the phosphosite (PS). F, Dissociation of peptides with arginine at P1 compared with peptides with alanine at P1 for both the phosphorylated (top) and unmodified (bottom) versions of the peptides. (***, p ≤ 0.001)