Figure 4.
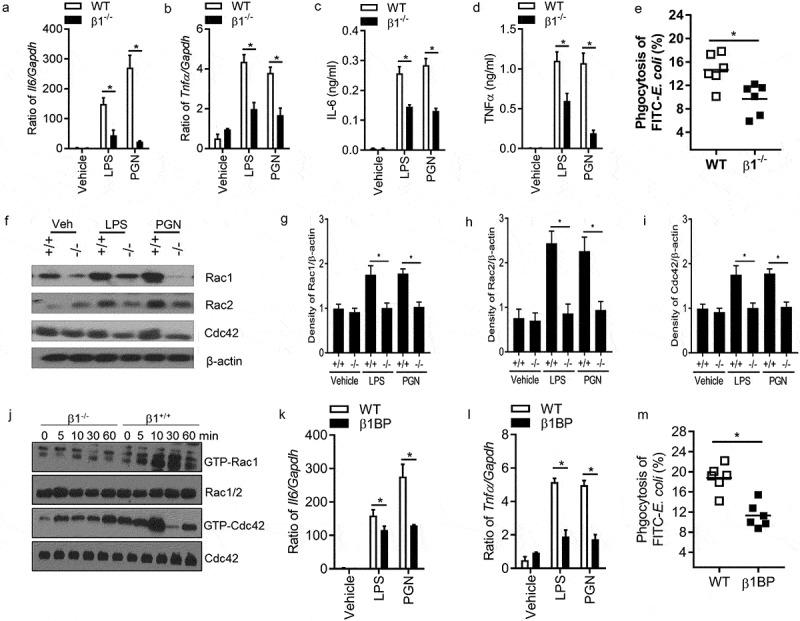
Ablation of Integrin β1 has defective responses to LPS and PGN in macrophages. Il6 (a) and Tnfα (b) mRNA expression were quantified by qPCR after LPS and PGN stimulation in WT and integrin β1-/- macrophages (n = 5, *p < 0.05). IL-6 (c) and TNFα (d) protein level were detected in the supernatant of WT and integrin β1-/- macrophages after LPS and PGN stimulation by ELISA (n = 5, *p < 0.05). Phagocytosis of integrin β1+/+ and integrin β1-/- macrophages to FITC conjugated E. coli was examined by FACS (e). Peritoneal macrophages from integrin β1-deficient and WT mice and FITC-labeled bacteria opsonized with normal mouse serum were mixed in suspension for 30 min at 37°C at a ratio of 1:100 (cell/bacteria). Phagocytosis was assessed by detecting the MFI of the cells after quenching by FACS. (n = 6, *p < 0.05). Total Rac1, Rac2 and Cdc42 protein in integrin β1-/- and integrin β1+/+ peritoneal macrophages were analyzed by western blot after LPS and PGN stimulation (f), and Rac1 (g), Rac2 (h) and Cdc42 (i) densities were quantified by comparing with β-actin with ImageJ software. (n = 3, *p < 0.05). The activity of Rac1, Rac2 and Cdc42 protein in integrin β1-/- and integrin β1+/+ peritoneal macrophages were also analyzed by western blot after LPS stimulation (j). Il6 (k) and Tnfα (l) mRNA expression of macrophages were quantified by qPCR with and without integrin β1 blocking peptide after LPS and PGN stimulation (n = 5, *p < 0.05). Phagocytosis of macrophages to FITC conjugated E. coli was examined with/without integrin β1 blocking peptide by FACS (m). (n = 5, *P < 0.05). Statistical comparisons among treatment groups were performed by randomized-design two-way ANOVA and followed by unpaired Student’s t-test for two groups using Prism software (Graph Pad Inc., La Jolla, CA). Statistical significance was defined as a P value of less than 0.05.
