Abstract
In most cases, sorafenib-resistant HCC cells exhibit significant mesenchymal phenotype and stemness features. In this context, tumor cells might undergo cell fate transition in response to sorafenib or other targeted drugs in the presence or absence of genetic mutations. Therefore, understanding the major characteristics of drug-resistant cells state helps to discover new treatments that overcome drug resistance. To note, little is known about the metabolic or microenvironmental aspects of the certain tumor cell states beyond the genome. This review mainly focuses on the underlying mechanisms of acquired sorafenib resistance based on CSCs and EMT models, which explain tumor heterogeneity and have been considered the major cause of secondary sorafenib resistance. In particular, it discusses how the tumor microenvironment and tumor metabolism regulate cell stemness, mesenchymal state, and sorafenib resistance through epigenetic regulations, and provides reliable targets that might have synergetic effect with sorafenib.
Keywords: Tumor metabolism, Tumor microenvironment, Hypoxia, Cancer stem cells, Epithelial-mesenchymal transition, Sorafenib resistance
1. Introduction
Liver cancer is the second-leading cause of cancer-related death globally, largely because of the limited number of effective interventions for advanced hepatocellular carcinoma (HCC) [1]. Sorafenib, a first-generation targeted therapy, was confirmed to be beneficial for patients with late-stage HCC [2,3]. Unfortunately, most patients did not experience a long-term benefit, largely because of the early occurrence of sorafenib resistance. New drug development has encountered huge obstacles in the next ten years since the approval of sorafenib. Until in 2017 and 2018, several new drugs were approved as first- or second-line targeted drugs for advanced HCC. Defining the underlying mechanisms of sorafenib resistance is still of great significance for other new targeted drugs.
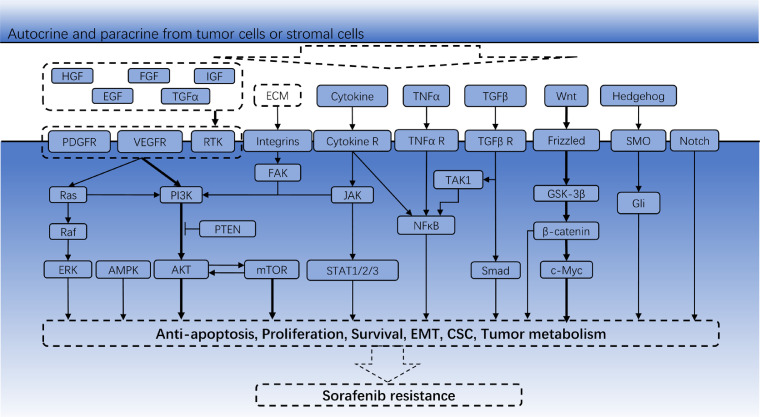
Genomic and transcriptional heterogeneity has been identified especially in patients with multifocal HCC, which is considered the major cause of treatment failures since both trunk and branch sorafenib-targeting mutations are low-frequency events in HCC [4]. Epithelial-mesenchymal transition (EMT) and cancer stem cell (CSC) are typical tumor heterogeneity models and contribute to phenotypic diversity of HCC cells. Stemness and mesenchymal features contributing to primary sorafenib resistance might be acquired at tumor initiation with the help of oncofetal proteins or pathogenic factors [5,6]. Acquired resistance is always established during long-term sorafenib exposure, whereby genomic instability serves as a platform in which random mutations occur in different tumor cells subsequently endowed with different fitness, and sorafenib itself as a selective force favors the outgrowth of drug-resistant subclones. In this context, cellular heterogeneity is characterized by molecular heterogeneity that compensates tumor cells for Raf kinase signaling blockade by sorafenib (Fig. 1). In this context, oncoprotein like phosphorylated ERK might be promising biomarker for sorafenib response [7]. On the other hand, HCC cells gradually transformed into a remarkable mesenchymal state, and Liver CSCs could be enriched following long-term sorafenib exposure in vivo and in vitro [8,9]. This indicates that tumor cells might undergo cell fate transition to become resistant to sorafenib (Fig. 2a-b). However, even liver CSCs displays heterogeneous sensitivity to sorafenib and EMT transformation can be canceled by sorafenib [9,10]. It is because CSCs themselves undergo clonal evolution and EMT can be induced by various signals (Fig. 2a).
Fig. 1.
Pathways involved in cell proliferation, EMT, CSCs and tumor metabolism in sorafenib resistance.
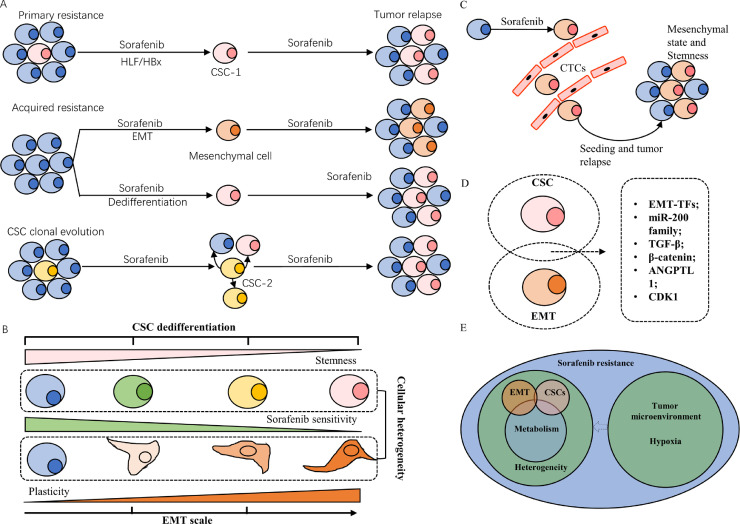
Fig. 2.
Cancer stem cells, Epithelial-mesenchymal transition and Sorafenib resistance. (a) The existence of CSCs with the help of HBx or oncoprotein HLF contributes to primary sorafenib resistance. Some HCC cells could induce EMT or dedifferentiation under long term exposure to sorafenib, acquiring stemness and plasticity and leading to second sorafenib resistance. Not all CSCs are naturally resistant to sorafenib and they also undergo clonal evolution and transform to be sorafenib resistant, especially. (b) CSCs dedifferentiation and EMT scale account for cellular heterogeneity within a tumor. Distinct tumor subpopulations exhibit diverse degrees of sensitivity to sorafenib. (c) HCC cell with mesenchymal states or stemness have higher invasive ability and become CTCs that have higher tumor-initiating ability to seed second tumors. (d) Mesenchymal HCC cells and liver stem cells share common gene signatures. (e) Schematic diagram of the relationship among tumor heterogeneity, tumor metabolism and tumor microenvironment. Abbreviation: CSC, cancer stem cell; EMT, epithelial-mesenchymal transition; CTCs, circulating tumor cells.
Stemness and mesenchymal states had been identified within a distinct group of EpCAM+ circulating tumor cells (CTCs), detecting which was proved to be advantageous for evaluating response to sorafenib (Fig. 2c) [11]. This highlights the importance of defining tumor cell states in monitoring sorafenib sensitivity and indicates that EMT and CSCs are not mutually exclusive. They share common gene signatures, most of which are EMT-inducing transcription factors (EMT-TFs) (Fig. 2d). Emerging studies suggested that EMT-TFs and pluripotency factors can regulate tumor metabolism in response to sorafenib [12,13]. Different EMT states and CSCs are localized in certain microenvironmental niches and closely in contact with different stromal cells [14,15]. Hence, this review will specifically focus on the metabolic changes and microenvironmental interplay in EMT transition or CSCs evolution beyond genome, which help us to have a comprehensive understanding of the relationship among tumor cell states, tumor heterogeneity, and sorafenib resistance (Fig. 2e).
2. Tumor microenvironment (TME) and sorafenib resistance
2.1. Sorafenib-induced hypoxia (SIH)
Sorafenib treatment resulted in decreased numbers of tumor vessels and pericyte depletion, and subsequent hypoxia that elicited EMT and resistance to sorafenib.[16] SIH promotes the nuclear accumulation and stabilization of HIF-1α and HIF-2α, and causes subsequent enhanced angiogenesis and transcription of oncogenes that enable HCC cells to adapt to sorafenib [17,18]. Moreover, sorafenib triggers the switch from HIF-1α- to HIF-2α-dependent pathways [19], making such adaptation stronger and fairly flexible. Collectively, HIF family plays central role in hypoxia-mediated sorafenib resistance (Fig. 3a), and increasing degradation of HIF proteins by small molecules restored sorafenib sensitivity in HCC [20,21]. From a CSC perspective, SIH and HIF family could enhance the stemness of HCC cells through promoting the expression of stemness-regulated genes and stem cell markers [22,23], or by downregulating the expression of AR [24]. As we have shown before, applying potent HIF-2α inhibitor or AR inhibitor can significantly enhance sorafenib efficacy in HCC [25,26]. A significant shift of blood supply from relying on angiogenesis to vessel co-option has been recognized in response to the anti-angiogenesis effect of sorafenib [27]. Researchers also identified high enrichment of CSCs in these vascular niches, and close interactions between CSCs and vascular niches mediated by exosomes via the exchange of growth and pro-angiogenic factors under hypoxia [28]. However, the role of such communication in promoting sorafenib resistance has not been exactly elucidated.
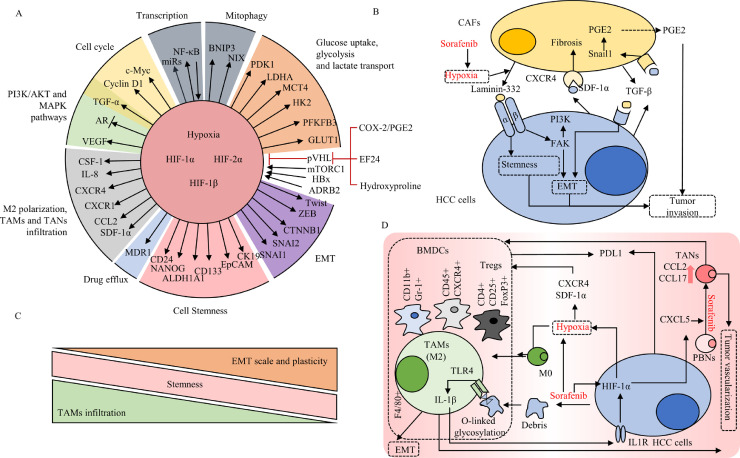
Fig. 3.
The role of hypoxia and stromal cells infiltration in sorafenib resistance. (a) The role of HIF family in hypoxia-mediated sorafenib resistance. (b) The interaction between CAFs and HCC cells under sorafenib-induced hypoxia. (c) Sorafenib-induced hypoxia and HCC cells debris shape an immunosuppressive HCC microenvironment by recruiting BMDCs, Tregs and TAMs, promoting M2 polarization and educating PBNs into TANs. (d) EMT process and tumor plasticity are negatively associated with the TAMs infiltration, while the stemness among different EMT intermediate states keep the same. Abbreviation: CAFs, cancer-associated fibroblasts; PBNs, peripheral blood neutrophils; BMDCs, bone marrow-derived cells; TAMs, tumor-associated macrophages; TANs, tumor-associated neutrophils; Tregs, T regulatory cells.
2.2. Stromal cells infiltration
The killing effect of certain anti-tumor drugs could be rendered in the presence of stromal cells, which was more pronounced for targeted drugs than for traditional chemotherapeutic drugs [29]. The infiltration of cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and tumor-associated neutrophils (TANs) have been proved to be correlated with sorafenib sensitivity (Fig. 3b) [30], [31], [32]. Those stromal cells have profound impact on regulating HCC cell states [6], [15]. Hepatic stellate cells (HSCs) can induce EMT process of sorafenib-resistant HCC cells by producing extracellular components, diffusible signaling molecules, and activating signals [33]. The distribution of TAMs shows consistence with progressive EMT states (Fig. 3c) [14]. It may due to that TAMs could induce EMT and increase stemness properties in HCC samples receiving sorafenib [34], however, the details of the communication between TAMs and HCC cells remain largely unknown.
The key process remains to be elucidated to explain how sorafenib promotes the infiltrations of stromal cells. Some studies pointed out the SIH could enhance the expression of cytokines and chemotactic factor like IL-1β and CXCL5 in HCC cells in a HIF-dependent manner [32,35]. Those factors attract peripheral blood neutrophils and educate them to become TANs, which then recruit TAMs and T-regulatory (Treg) cells that together induce tumor vascularization to survive the hypoxia [32]. SIH also promotes immunosuppression, characterized by increased intra-tumoral expression of programmed death ligand-1 (PD-L1) and accumulation of Treg cells and TAMs (Fig. 3d) [36]. In this context, SIH counteracts the tumor cell killing effect of immune cells, leading to tumor relapse.
2.3. Extracellular vesicles
Drug-resistant cells benefited from surrounding drug-sensitive cells in response to targeted drug whereby “secretomes” derived from drug-sensitive cells attract drug-resistant cells and foster their outgrowth [37]. Extracellular vesicles (EVs) might be the major part of these “secretomes”, and are originated from either stromal cells or tumor cells. Exosomes and their cargos such as miRNAs, modulate sorafenib sensitivity in vivo and in vitro [38]. Little publications are available in the literature that address the mechanisms of exosome-mediated sorafenib resistance. Recent studies suggested that hypoxia and HIF family increased the generation and secretion of exosomes and induced the transcription of exosomal cargos, especially microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) [39]. A large amount of exosomal miRNAs and lncRNAs could promote EMT and transfer mesenchymal phenotype to the recipient tumor cell [40]. Moreover, as discussed previously, exosomes mediate the communication between stem cells and vascular niches and between CAFs and mesenchymal cells. Collectively, we assume that exosomes might mediate sorafenib resistance by promoting CSC phenotypes, EMT, or adaptation to hypoxic conditions.
3. Tumor metabolism and sorafenib resistance
3.1. Metabolic switch of glucose metabolism and alternative energy sources
Metabolic processes are demonstrated to exhibit consistent prognostic patterns, and they are associated with the sensitivity of drugs in clinical use. 2-deoxy-d-glucose, a common glycolytic inhibitor, could drastically inhibit the growth of sorafenib-resistant cells [41]. Glucose uptake and lactate export have also been enhanced in response to sorafenib [42]. Key enzymes in glycolysis including PFKFB3, HK2, and PKM2 have been demonstrated overexpression in HCC patients or sorafenib-resistant HCC cell lines to increase glycolytic flux and enhance glycolysis [43]. Silencing these enzymes has shown synergetic effect with sorafenib [44], [45], [46]. In addition, glycolysis under hypoxic environments exhibits high dependency on HIF family, especially in aggressive HCC [47]. Interestingly, these metabolic enzymes even could directly bind to HIF-1α or forms a positive feedback loop with HIF-1α at transcriptional level [45,48]. Inhibiting glycolysis by specific molecule or by targeting key enzymes of glycolysis is effective strategy to attenuates sorafenib resistance specially under SIH.
Metabolic switch of glucose metabolism might be more pronounced in CSCs. Glucose uptake is remarkably increased in liver CSCs via the preferential expression of the certain glucose transporters, inhibition of which can increase the sensitivity to sorafenib in vivo [49]. Low levels of total and phosphorylated AMPK, which is a low energy sensor that favors oxidative phosphorylation (OXPHOS), had been detected in sorafenib-resistant stem-like HCC cells and promotes the expression of stemness-related genes through regulating HIF-1α level [50]. Those suggest that liver CSCs are highly dependent on glycolysis, but we cannot then exclude the potential role of OPXHOS in stemness regulation. Mitophagy has been proved to regulate stemness of liver CSCs [51]. It has a dual function in modulating the sensitivity of sorafenib in HCC. Mild mitophagy mediated drug resistance by degradation of sorafenib-damaged mitochondrial while excessive mitophagy exacerbated sorafenib-induced apoptosis [52,53]. Collectively, mitochondrial function and OXPHOS are involved in the regulation of CSC-mediated sorafenib resistance. SIRT1/MRPS5 axis bridges the stemness properties and OXPHOS regulation. Moreover, a switch from OXPHOS to glycolysis in response to hypoxia depends on the acetylation status of MRPS5 protein [51], representing a metabolic plasticity in liver CSCs. Altogether, energy addiction is a striking characteristics of liver CSCs, featured by both addicted glycolysis and sustained OXPHOS.
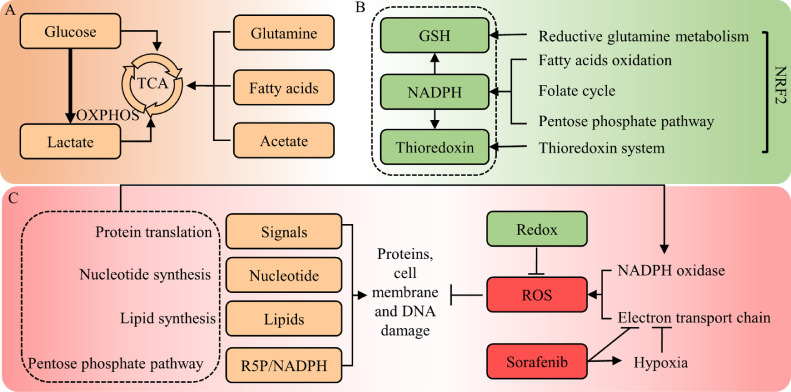
Given that glucose flows more to the glycolysis pathway in CSCs, there might be alternative energy sources for liver CSCs to complete krebs cycle (TCA cycle) and fuel OXPHOS (Fig. 4a). Fatty acid addiction and enhanced fatty acid oxidation (FAO) have been observed during CTNNB1-mutated HCC tumorigenesis [54], and can be activated by NANOG to support the self-renewal ability of CSCs and sorafenib resistance [13]. Glutamate oxidation becomes the main energy source for OXPHOS in HCC cell line under aglycemia [55]. Overall, these findings revealed the plasticity of energy metabolism in liver CSCs and their contribution to sorafenib resistance. NANOG, MYC, and CTNNB1 are key genes regulating the crosstalk of cancer stemness, energy metabolism, and sorafenib resistance in HCC.
Fig. 4.
Metabolic homeostasis in sorafenib resistance. (a) OXPHOS is sustained in liver CSCs, and glutamine, fatty acids and acetate could be alternative energy sources to fuel HCC cells under sorafenib-induced hypoxia and relative glucose deprivation. (b) Redox production including GSH, NAPDH and thioredoxin involves multiple metabolic pathways and plays central role in against sorafenib-induced oxidative stress, especially in EMT process. NRF2 plays the key role in (c) Enhanced proteins, lipids and nucleotides biosynthesis are crucial to maintain cell structure, support DNA repair and supply pro-survival growth signals. Abbreviations: OXPHOS, Oxidative phosphorylation; TCA, tricarboxylic acid cycle; GSH, glutathione; NADPH, nicotinamide adenine dinucleotide phosphate; R5P, Ribose 5-phosphate; ROS, reactive oxygen species.
3.2. Lactate links tumor metabolism to TME
Lactate is the main byproduct of glycolysis. Studies have shown that tumor cells can utilize lactate in the TME through multiple pathways to survive the targeted drugs. A lactate shuttling had been identified between CAFs and tumor cells, which help tumor cells remove excess lactate [56]. Moreover, accumulated lactate can act as a signaling molecule and directly stimulate CAFs to secrete growth factors and cytokines, that can be utilized by tumor cells to establish adaptive resistance to targeted drugs [57]. Similar phenomena may also occur in sorafenib-resistant HCC, but there are currently no relevant data clarifying this possibility. The co-existence of hypoxic and normoxic regions has been identified inside tumors in terms of the relative proximity to blood vessels in vivo [58]. Those two regions surprisingly form metabolic symbiosis by shuttling lactate via distinct expression patterns of glucose and lactate transporters or metabolic enzymes in a HIF-1α-dependent manner. This phenomenon is consistent with the idea that tumor cells maintain high rates of both glycolysis and OXPHOS, whereas addiction to glycolysis occurs only in the core of the tumor under hypoxia. Above all, lactate or other substances have more diverse functions than just metabolites in the development of sorafenib resistance.
3.3. Sorafenib-induced oxidative stress and reactive oxygen species (ROS) control
As for the reliance of HCC cells on oxidative stress response for growth advantage and sorafenib sensitivity, sorafenib itself exerts a positive influence on ROS production in HCC by targeting mitochondrial electron transport chain complexes and ATP synthases [59]. Meanwhile, using dichloroacetate (DCA), a pyruvate dehydrogenase kinase (PDK) inhibitor, reversed sorafenib resistance in highly glycolysis-addicted HCC cells. However, such a reversal is not attributed to the suppression of glycolysis nor the additional inhibition of ERK signaling, but enhanced ROS production and ROS-induced apoptosis [60]. Thus, ROS control plays a crucial role in the development of sorafenib resistance in HCC.
Glutathione (GSH) synthesis plays central role in ROS control. β-catenin and c-Myc are proved to be the key proteins in GSH-dependent stemness maintenance and sorafenib resistance [61,62]. In contrast, decreased glutaminolysis mediated by glutamine synthetase (GS) contributes to enhanced sensitivity to sorafenib in HCC [63]. Tumor cells undergoing EMT acquire metastasis potential and escape from anoikis, a cell death program induced by ATP deficiency due to ECM detachment. Moreover, one study assessed the correlation between mesenchymal states scored by several sets of gene signatures and drug AUC, confirming that the contribution of the mesenchymal state to therapeutic resistance is highly dependent on GPX4, a glutathione peroxidase that act against lipid peroxidation and ferroptosis, a form of oxidative necrosis [12]. Ferroptosis can be induced by several compounds including sorafenib. Activation of the p62/Keap1/NRF2 pathway protected against sorafenib-induced ferroptosis by directly modulating ferrous iron (Fe2+) metabolism genes in HCC [64]. NRF2 is a master regulator of redox homeostasis and mediates the overexpression and activation of antioxidants including MTIG, TXNRD1, MTHFD1L, and NADPH, all of which have been proved to overcome sorafenib-induced oxidative stress [65], [66], [67], [68]. In this context, antioxidants mediate EMT-induced sorafenib resistance by supporting the high ATP consumption in HCC. FGF19/FGFR4 axis recently becomes a promising target for the treatment of HCC. It has been reported that FGF19/FGFR4 inhibited sorafenib-induced ROS generation and apoptosis [69], and was the upstream of NRF2 [70]. But more studies are needed to demonstrate the potential role of FGF19/FGFR4 in NRF2-mediated anti-ferroptosis. Altogether, ROS-mediated damage potentiates the antitumor effect of sorafenib and ROS control plays key role in cell state regulation and sorafenib resistance (Fig. 4b).
3.4. Enhanced protein translation and lipid synthesis
Sorafenib-resistant HCC cells showed constitutive activation of mTOR pathway. mTORC1/RPS6 controls global biosynthesis and had been found to be correlated with responsiveness of HCC to sorafenib[71]. For example, it promotes the translation of HIF-1α, and melatonin had been found to inhibit mTORC1-mediated HIF-1α synthesis and improve sorafenib sensitivity in HCC cell lines [72]. mTORC1 also upregulates expression of enzymes and glucose flux of pentose phosphate pathway (PPP), a parallel metabolic pathway to glycolysis. PPP produces precursors for many biosynthesis pathways to support tumor growth. Lower expression of G6PD, the first and rate-limiting enzyme of PPP, had been significantly associated with sorafenib benefit in patients. Furthermore, the common downregulation of PTEN in HCC was attributed to increased G6PD expression levels [73].
Another important role of PPP is to produce NADPH as substrate for fatty acid and lipid synthesis. A metabolic shift toward lipid synthesis has been observed after the withdrawal of anti-angiogenic treatment including sorafenib in PDX models. Inhibiting fatty acid synthase (FASN) blocked lipid synthesis and restored the anti-tumor effect of sorafenib [74]. Mutation of two AMPK phosphorylation sites within ACC1 enhanced de novo lipogenesis (DNL), and inhibition of ACC1 improved sorafenib efficacy in a rat model [75]. One study revealed upregulated SCD1 in sorafenib-resistant HCC cell lines, and its expression predicted clinical benefit for sorafenib in patients. Adding exogenous oleic acid, one of the enzymatic products of SCD1, to HCC cells, rescued the effect of SCD inhibitor on sorafenib sensitization [76]. Another product of PPP is ribose 5-phophate that is utilized in nucleotides synthesis, the role of which in sorafenib resistance, however, has not be studied. Collectively, enhanced NAPPDH, protein, and lipid synthesis are crucial to meet the need of biomass to support cell growth in response to sorafenib (Fig. 4c).
4. Epigenetic regulation links microenvironmental or metabolic changes to cell state transition
4.1. Stromal cell infiltration and chromatin remodeling
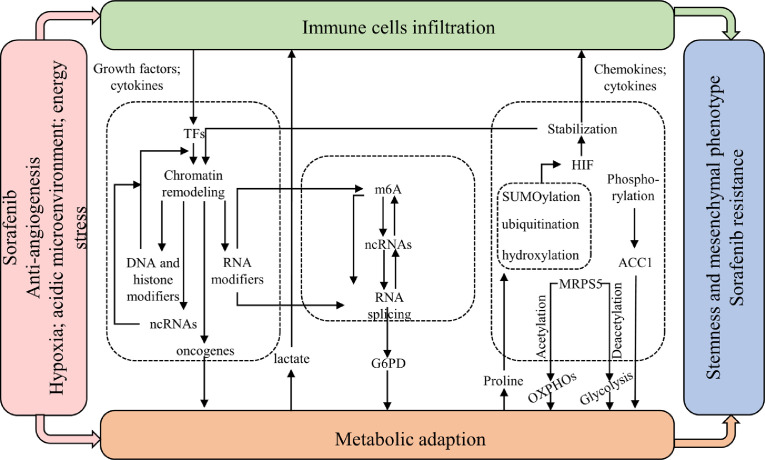
Researchers have identified different EMT tumor subpopulations which are spatially organized in particular microenvironments with the infiltration of specific stromal populations, especially macrophages [14]. They applied ATAC-seq analysis and unraveled stepwise and very specific chromatin remodeling in the different EMT states. But they didn't uncover mechanisms underlying how macrophages infiltration facilitates chromatin remodeling in tumor cells. As we discussed before, infiltration of stromal cells might be a striking characteristic of EMT-related sorafenib resistance in HCC. Cytokines and chemokines secreted from immune cells such as TGF-β, IL-6, HGF, and COX2 might be responsible for the stroma-mediated cell state transition and sorafenib resistance, given that TGF-β/SMAD, IL-6/STAT3, HGF/MET, COX2/HIF-1α and TNF-α/NF-κB pathways have been widely recognized as inducers of EMT, stemness, and sorafenib resistance [18,22,29,34]. Activation of these pathways in HCC leads to global enhanced transcriptional activity including those DNA and histone methylation modifiers that further enhance transcription of oncogenes such as IGF2, another key factor of cell state regulation and sorafenib resistance (Fig. 5) [77]. It has been reported that epigenetic reconditioning by using demethylating compound 5-azacytidine (5-AZA) improved sorafenib sensitivity in HCC [78]. HDAC inhibitors also show synergetic effect with sorafenib [79], indicating that histone deacetylases might be involved in sorafenib resistance. These preclinical data have proved that targeting DNA or histone modifiers might be an effective strategy to overcome sorafenib resistance in HCC.
Fig. 5.
Epigenetic regulation links microenvironmental or metabolic changes to cell state transition. Abbreviation: m6A, N6-methyladenosine; ncRNAs, non-coding RNAs.
4.2. Metabolic changes and post-translational modifications
Hypoxia and HIF family mediate the upregulation and secretion of cytokines and chemokines from stromal cells under sorafenib treatment. We revealed that metabolic changes under SIH directly induced epigenetic regulations (Fig. 5). Metabolites like lactate could act as a direct signal instructing CAFs to produce HGF for tumors to survive the killing effect of sorafenib [57]. Recent studies also report that lactate and acetyl-CoA could act as substrates for histone modifications that epigenetically promote EMT and M2 polarization under hypoxia [80,81]. On the other hand, metabolic enzymes also have a direct role in regulating transcription and translation in response to hypoxia and sorafenib treatment [45]. Proline and hydroxyproline metabolism could modulate HIF1α stability through inhibiting hydroxylation of HIF1α protein and subsequent pVHL-mediated degradation [82]. Other post-translational modifications of HIF family including SUMOylation and ubiquitination have also been well studied under SIH in HCC [20,23]. SIRT1-mediated deacetylation controls the dual function of MRPS5 in regulating the switch of mitochondrial-dependent energy supply to glycolysis in liver CSCs under hypoxia [51]. Attenuated phosphorylation of ACC1 by AMPK improved tumor survival under sorafenib treatment [75]. These findings confirm that post-translational modification is an important pathway for regulating tumor adaptation to sorafenib during metabolic reprogramming.
4.3. Post-transcriptional regulation
ncRNAs are emerging key regulators of post-transcriptional activity in cancers. miRNAs are most frequently studied and some of them have been proved to be significantly dysregulated in HCC, promoting tumor progression and sorafenib resistance [83,84]. Consistent with the idea that certain cell states determine drug sensitivity, miRNAs, as well as other ncRNAs including lncRNAs that mostly act as sponges of miRNAs, modulated sorafenib sensitivity through regulating EMT and stemness in HCC [85]. There are no studies reporting the role of circular RNAs (circRNAs) in sorafenib resistance yet, however, circRNA was involved in regulating stemness features of HCC cells [86]. In addition, acidic microenvironment, energy stress, and immune cell infiltration enhanced the transcription of ncRNAs in HCC cells, and promoted EMT, stemness, and angiogenesis in a HIF-dependent manner or with the help of stroma-derived growth factors [15,27,87,88]. LncRNAs could also directly bind to chromatin-remodeling complex to increase stemness features of liver CSCs [89], and could be transcriptionally driven by EMT-TFs to regulate EMT process in turn [90]. These findings shed new insights into ncRNA-mediated interplay among microenvironment, metabolism, and tumor cell states in HCC, and provide ideas for further research of ncRNA-mediated cell state transition in sorafenib resistance.
RNA processing has attracted much attention in tumor regulation and endows HCC cells with molecular heterogeneity at post-transcriptional level, which is the key factor modulating sorafenib sensitivity. One studied revealed that splicing factors and mRNA splicing are involved in sorafenib resistance through regulating glucose metabolism [73]. Still, little is known about the role of RNA processing in cell state transition and sorafenib resistance. ncRNAs could directly bind to splicing factor and RNA helicases, while m6A and splicing factors regulates processing and splicing of ncRNAs. In this context, ncRNAs together with RNA processing establish a complex epigenetic regulation network, which might have great impact on diverse responses of tumor cells towards treatment (Fig. 5).
5. Conclusion and outstanding questions
CSCs and EMT models provide cellular and molecular heterogeneity that endow HCC cells with diverse plasticity and fitness advantages in response to sorafenib. One reason for the great attention paid to immunotherapy is that it exerts tumor killing effect at cell-to-cell level unlike sorafenib, regardless of the compensation of the intracellular signaling pathway network. In this context, targeting tumor cell with certain states, namely mesenchymal and stemness states which are associated with adaptive resistance to sorafenib, might be a promising combinational strategy with sorafenib or other TKIs. Hence, we elucidated the striking features of HCC cells with certain states from microenvironmental and metabolic perspectives. We found that liver CSCs and mesenchymal cells were in close contact with the stroma under sorafenib treatment. Extracellular components, diffusible signaling molecules, and activating signals mediated such communication in an autocrine, paracrine, or EVs-dependent manner. With respect to metabolic alterations, liver CSCs and mesenchymal cells exhibit high dependencies on energy supply, redox homeostasis, and enhanced biosynthesis. Key regulators like NANOG, c-MYC, andβ-catenin mediated the interplay between tumor metabolism and cell fate transition. Moreover, sorafenib-induced hypoxia and HIF family were common causes of these metabolic and microenvironmental changes, and play central roles in regulating stem cell specification, EMT, metabolic reprogramming, vascularization, immune suppression, and their crosstalk in sorafenib resistance. At last, we found that epigenetic alterations are frequent events within HCC cells in response to sorafenib, linking microenvironmental or metabolic changes to cell state transition. We also summarized the preclinical practices of drugs in combination with sorafenib, hoping to provide future directions for the development of new treatment for HCC patients (Table 1).
Table 1.
Preclinical practices of drugs in combination with sorafenib in HCC.
| Drug | Target | Effect of the drug | Reference |
|---|---|---|---|
| Hypoxia inhibitors | |||
| EF24 | HIF-1α | promoting VHL-dependent HIF-1α degradation and NF-κB inactivation | [20] |
| PT-2385 | HIF-2α | suppressing HIF-2α, increasing AR and suppressing downstream pSTAT3/pAKT/pERK pathways. | [25] |
| ICI-118551 | ADRB2 | inhibiting ADRB2 signaling and enhancing autophagic HIF1α degradation | [21] |
| Meloxicam | COX2 | promoting VHL-dependent HIF-2α degradation, and inhibiting HIF-2α nuclear translocation | [18] |
| Celecoxib | |||
| 2-ME2 | HIF-1α | reducing the expression of both HIF-1α and HIF-2α | [19] |
| HIF-2α | |||
| Melatonin | HIF-1α | inhibiting mTORC1/HIF-1α and hypoxia-mediated mitophagy | [72] |
| Stemness inhibitors | |||
| ATRA | AKT | reducing the EpCAM+ tumor cell population | [9] |
| Nifuroxazide | STAT3 | blocking activation of STAT3 and expression of CD133 and HIF-1α proteins | [22] |
| ASC-J9 | AR | blocking activation of STAT3 | [26] |
| SSI-4 | SCD1 | inducing ER stress and suppressing liver CSCs | [76] |
| Tumor microenvironment modulators | |||
| AMD3100 | CXCR4 | reducing Gr-1(+) myeloid cell infiltration | [30,36] |
| Zoledronic acid | TAMs | depletion of macrophages and inhibiting tumor angiogenesis | [31] |
| Clodrolip | |||
| Anti-Ly6G | TANs | depletion of TANs and inhibiting neovascularization | [32] |
| Metabolic modulators | |||
| Etomoxir | CPT1 | inhibition of FAO in liver CSCs | [13] |
| 2-DG | G6P | reducing glucose uptake and cellular ATP levels | [41,43,62] |
| Aspirin 3PO |
PFKFB3 | inhibition of PFKFB3 and glycolysis | [44] |
| PB2 | PKM2 | suppressing glucose uptake and aerobic glycolysis | [45] |
| DCA | PDK | reducing lactate production and increasing ROS | [60] |
| 3BP | HK2 | inhibiting glycolysis | [46] |
| A-769662 FCCP |
AMPK | Activating AMPK and decreased the expression of stemness markers | [50,53] |
| Ketoconazole | COX2 | promoting mitophagy and mitochondrial dysfunction | [52] |
| BPTES | GLS1 | inhibiting glutaminolysis | [62] |
| 10058-F4 | c-Myc | inhibiting c-Myc | [62] |
| ND-654 | ACC1 | inhibiting hepatic DNL | [75] |
| Oxidative stress inducers | |||
| Alkaloid trigonelline | NRF2 | inducing ferroptosis | [64] |
| ATRA PPG |
MT1G | increasing GSH depletion and ferroptosis | [65] |
| OT | TKT | increasing ROS accumulation | [66] |
| MTX | Folate | inhibition of the folate cycle | [67] |
| AUR | TXNRD1 | increasing ROS accumulation | [68] |
| Ponatinib | FGFR4 | enhancing ROS-associated apoptosis | [69] |
| Epigenetic modulators | |||
| 5-AZA | - | demethylation of DNA | [78] |
| Panobinostat | HDAC | increasing histone H3 and HSP90 acetylation | [79] |
| Others | |||
| DR | KRAS | suppressing RAF/ERK and PI3K/AKT signaling | [83] |
Abbreviations: 2-ME2, 2-Methoxyestradiol; ATRA, all-trans retinoic acid; 2-DG, 2-deoxy-d-glucose; 3PO, 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one; PB2, proanthocyanidin B2; DCA, dichloroacetate; 3BP, 3-bromopyruvate; FCCP, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone; PPG, propargylglycine; OT, oxythiamine; MTX, methotrexate; AUR, auranofin; 5-AZA, 5-azacytidine; DR, Deltarasin.
Future research needs to improve three aspects: (1) undiscovered associations between tumor metabolism, tumor microenvironment, tumor cell status, and sorafenib sensitivity. (2) advances in liquid biopsy to detect the mesenchymal or stemness states of tumor cells, which allow researchers to better discriminate and monitor sorafenib sensitivity among HCC patients. (3) new strategies targeting sorafenib-resistant tumors based on their high dependencies on tumor microenvironment and metabolic reprogramming.
6. Search strategy and selection criteria
Data for this Review were identified by searches of PubMed, and references from relevant articles using the search terms “sorafenib” and “hepatocellular carcinoma”. Most of references are articles published in English between 2008 and 2019 were included, while few of them are reviews to explain well-known concepts.
SJ Xia and JJ Xu provided the idea of the article. SJ Xia researched data and wrote the article. Y Pan, YL Liang, JJ Xu and XJ Cai reviewed and edited the manuscript before submission. JJ Xu and XJ Cai also made substantial contributions to the discussion of content.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Acknowledgment
The authors thank Joe Barber Jr., PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
National Natural Science Foundation of China under Grant no. 81772546 (to Xiujun Cai); Zhejiang Provincial Natural Science Foundation of China under Grant no. LQ19H160026 (to Junjie Xu) and no. LGF18H160011 (to Yuelong Liang); Special fund for basic scientific research operating expenses of Zhejiang University under Grant no. 2019XZZX005-4-05 (to Yuelong Liang); Hepatobiliary and Pancreatic Cancer Research of Hubei Chen Xiaoping Science and Technology Development Foundation under Grant no. CXPJJH11900001-2019308 (to Junjie Xu). The funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
Contributor Information
Junjie Xu, Email: walter235@zju.edu.cn.
Xiujun Cai, Email: srrsh_cxj@zju.edu.cn.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet‐Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Cheng A.-L., Kang Y.-K., Chen Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3.Llovet J.M., Ricci S., Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Xu L.X., He M.H., Dai Z.H. Genomic and transcriptional heterogeneity of multifocal hepatocellular carcinoma. Ann Oncol. 2019;30:990–997. doi: 10.1093/annonc/mdz103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang D.-M., Sun W., Zhou T. Oncofetal HLF transactivates c-Jun to promote hepatocellular carcinoma development and sorafenib resistance. Gut. 2019;68:1858. doi: 10.1136/gutjnl-2018-317440. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Hao X., Sun R., Wei H., Tian Z. Natural killer cell-derived interferon-gamma promotes hepatocellular carcinoma through the epithelial cell adhesion molecule-epithelial-to-mesenchymal transition axis in hepatitis b virus transgenic mice. Hepatology. 2019;69:1735–1750. doi: 10.1002/hep.30317. [DOI] [PubMed] [Google Scholar]
- 7.Liang Y., Chen J., Yu Q. Phosphorylated ERK is a potential prognostic biomarker for Sorafenib response in hepatocellular carcinoma. Cancer Med. 2017;6:2787–2795. doi: 10.1002/cam4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Malenstein H., Dekervel J., Verslype C. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013;329:74–83. doi: 10.1016/j.canlet.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Guan D.X., Shi J., Zhang Y. Sorafenib enriches epithelial cell adhesion molecule-positive tumor initiating cells and exacerbates a subtype of hepatocellular carcinoma through TSC2-AKT cascade. Hepatology. 2015;62:1791–1803. doi: 10.1002/hep.28117. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y.L., Lv J., Ye X.L. Sorafenib inhibits transforming growth factor beta1-mediated epithelial-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology. 2011;53:1708–1718. doi: 10.1002/hep.24254. [DOI] [PubMed] [Google Scholar]
- 11.Guo W., Sun Y.-F., Shen M.-N. Circulating Tumor Cells with Stem-Like Phenotypes for Diagnosis, Prognosis, and Therapeutic Response Evaluation in Hepatocellular Carcinoma. Clin Cancer Res. 2018;24:2203. doi: 10.1158/1078-0432.CCR-17-1753. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan V.S., Ryan M.J., Dhruv H.D. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C.-L., Kumar Uthaya, Dinesh B., Punj V. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016;23:206–219. doi: 10.1016/j.cmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastushenko I., Brisebarre A., Sifrim A. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463. doi: 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S.L., Yin D., Hu Z.Q. A positive feedback loop between cancer stem-like cells and tumor-associated neutrophils controls hepatocellular carcinoma progression. Hepatology. 2019;70:1214–1230. doi: 10.1002/hep.30630. [DOI] [PubMed] [Google Scholar]
- 16.Cooke V.G., LeBleu V.S., Keskin D. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Z., Liu T., Chen J. HIF-1alpha-induced RIT1 promotes liver cancer growth and metastasis and its deficiency increases sensitivity to sorafenib. Cancer Lett. 2019;460:96–107. doi: 10.1016/j.canlet.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Dong X.-F., Liu T.-Q., Zhi X.-T. COX-2/PGE2 axis regulates HIF2α activity to promote hepatocellular carcinoma hypoxic response and reduce the sensitivity of sorafenib treatment. Clin Cancer Res. 2018;24:3204–3216. doi: 10.1158/1078-0432.CCR-17-2725. [DOI] [PubMed] [Google Scholar]
- 19.Ma L., Li G., Zhu H. 2-Methoxyestradiol synergizes with sorafenib to suppress hepatocellular carcinoma by simultaneously dysregulating hypoxia-inducible factor-1 and -2. Cancer Lett. 2014;355:96–105. doi: 10.1016/j.canlet.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y., Zheng T., Song R. Hypoxia‐mediated sorafenib resistance can be overcome by EF24 through Von Hippel‐Lindau tumor suppressor‐dependent HIF‐1α inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857. doi: 10.1002/hep.26224. [DOI] [PubMed] [Google Scholar]
- 21.Wu F.Q., Fang T., Yu L.X. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1alpha. J Hepatol. 2016;65:314–324. doi: 10.1016/j.jhep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Won C., Kim B.H., Yi E.H. Signal transducer and activator of transcription 3‐mediated CD133 up‐regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015;62:1160–1173. doi: 10.1002/hep.27968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui C.-P., Wong C.C.-L., Kai A.K.-L. SENP1 promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation and SENP1/HIF-1α positive feedback loop. Gut. 2017;66:2149. doi: 10.1136/gutjnl-2016-313264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Y., Sun Y., Liu G. Androgen receptor (AR)/miR-520f-3p/SOX9 signaling is involved in altering hepatocellular carcinoma (HCC) cell sensitivity to the Sorafenib therapy under hypoxia via increasing cancer stem cells phenotype. Cancer Lett. 2019;444:175–187. doi: 10.1016/j.canlet.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Xu J., Zheng L., Chen J. Increasing AR by HIF-2alpha inhibitor (PT-2385) overcomes the side-effects of sorafenib by suppressing hepatocellular carcinoma invasion via alteration of pSTAT3, pAKT and pERK signals. Cell Death Dis. 2017;8:e3095. doi: 10.1038/cddis.2017.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Lin H., Li G. Sorafenib with ASC-J9(®) synergistically suppresses the HCC progression via altering the pSTAT3-CCL2/Bcl2 signals. Int J Cancer. 2017;140:705–717. doi: 10.1002/ijc.30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuczynski E.A., Yin M., Bar-Zion A. Co-option of liver vessels and not sprouting angiogenesis drives acquired sorafenib resistance in hepatocellular carcinoma. JNCI: J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao H., Liu N., Lin M.C., Zheng J. Positive feedback loop between cancer stem cells and angiogenesis in hepatocellular carcinoma. Cancer Lett. 2016;379:213–219. doi: 10.1016/j.canlet.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Straussman R., Morikawa T., Shee K. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Huang Y., Reiberger T. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal‐derived factor 1 alpha/C‐X‐C receptor type 4 axis and myeloid differentiation antigen–positive myeloid cell infiltration in mice. Hepatology. 2014;59:1435–1447. doi: 10.1002/hep.26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W., Zhu X.-D., Sun H.-C. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S.-L., Zhou Z.-J., Hu Z.-Q. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 33.Azzariti A., Mancarella S., Porcelli L. Hepatic stellate cells induce hepatocellular carcinoma cell resistance to sorafenib through the laminin‐332/α3 integrin axis recovery of focal adhesion kinase ubiquitination. Hepatology. 2016;64:2103–2117. doi: 10.1002/hep.28835. [DOI] [PubMed] [Google Scholar]
- 34.Fan Q.-M., Jing Y.-Y., Yu G.-F. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial–mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Zhang Q., Lou Y. Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial–mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018;67:1872–1889. doi: 10.1002/hep.29681. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Ramjiawan R.R., Reiberger T. CXCR4 inhibition in tumor microenvironment facilitates anti‐programmed death receptor‐1 immunotherapy in sorafenib‐treated hepatocellular carcinoma in mice. Hepatology. 2015;61:1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obenauf A.C., Zou Y., Ji A.L. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature. 2015;520:368. doi: 10.1038/nature14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu Z., Wu J., Wu J., Luo D., Jiang C., Ding Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res. 2016;35:159. doi: 10.1186/s13046-016-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X.-P., Wang C.-Y., Jin X.-H. Acidic microenvironment up-regulates exosomal miR-21 and miR-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 2019;9:1965–1979. doi: 10.7150/thno.30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L., Guo P., He Y. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-0534-9. 513-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes R., Wani N.A., Ghoshal K., Jacob S.T., Motiwala T. Sorafenib and 2-deoxyglucose synergistically inhibit proliferation of both sorafenib-sensitive and-resistant HCC cells by inhibiting ATP production. Gene Expr. 2017;17:129–140. doi: 10.3727/105221616X693855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Q., Li J., Xing J. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol. 2014;61:859–866. doi: 10.1016/j.jhep.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 43.Wong T.L., Ng K.Y., Vin Tan K. CRAF methylation by PRMT6 regulates aerobic glycolysis driven hepatocarcinogenesis via ERK-dependent PKM2 nuclear relocalization and activation. Hepatology. 2019 doi: 10.1002/hep.30923. [DOI] [PubMed] [Google Scholar]
- 44.Li S., Dai W., Mo W. By inhibiting PFKFB3, aspirin overcomes sorafenib resistance in hepatocellular carcinoma. Int J Cancer. 2017;141:2571–2584. doi: 10.1002/ijc.31022. [DOI] [PubMed] [Google Scholar]
- 45.Feng J., Wu L., Ji J. PKM2 is the target of proanthocyanidin B2 during the inhibition of hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:204. doi: 10.1186/s13046-019-1194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo J.-J., Yu S.J., Na J. Hexokinase-II inhibition synergistically augments the anti-tumor efficacy of sorafenib in hepatocellular carcinoma. Int J Mol Sci. 2019;20:1292. doi: 10.3390/ijms20061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamaguchi T., Iizuka N., Tsunedomi R. Glycolysis module activated by hypoxia-inducible factor 1alpha is related to the aggressive phenotype of hepatocellular carcinoma. Int J Oncol. 2008;33:725–731. [PubMed] [Google Scholar]
- 48.Long Q., Zou X., Song Y., Duan Z., Liu L. PFKFB3/HIF-1α feedback loop modulates sorafenib resistance in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2019;513:642–650. doi: 10.1016/j.bbrc.2019.03.109. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H.L., Wang M.D., Zhou X. Blocking preferential glucose uptake sensitizes liver tumor-initiating cells to glucose restriction and sorafenib treatment. Cancer Lett. 2017;388:1–11. doi: 10.1016/j.canlet.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 50.Bort A., Sanchez B.G., Mateos-Gomez P.A., Vara-Ciruelos D., Rodriguez-Henche N., Diaz-Laviada I. Targeting AMP-activated kinase impacts hepatocellular cancer stem cells induced by long-term treatment with sorafenib. Mol Oncol. 2019;13:1311–1331. doi: 10.1002/1878-0261.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei Z., Jia J., Heng G. Sirtuin-1/Mitochondrial ribosomal protein S5 axis enhances the metabolic flexibility of liver cancer stem cells. Hepatology. 2019;70:1197–1213. doi: 10.1002/hep.30622. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y., Chen H.N., Wang K. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 2019;70:66–77. doi: 10.1016/j.jhep.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 53.Song J., Zhao W., Lu C., Shao X. LATS2 overexpression attenuates the therapeutic resistance of liver cancer HepG2 cells to sorafenib-mediated death via inhibiting the AMPK-Mfn2 signaling pathway. Cancer Cell Int. 2019;19 doi: 10.1186/s12935-019-0778-1. 60-60. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Senni N., Savall M., Cabrerizo Granados D. β-catenin-activated hepatocellular carcinomas are addicted to fatty acids. Gut. 2019;68:322. doi: 10.1136/gutjnl-2017-315448. [DOI] [PubMed] [Google Scholar]
- 55.Jezek J., Plecita-Hlavata L., Jezek P. Aglycemic HepG2 cells switch from aminotransferase glutaminolytic pathway of pyruvate utilization to complete krebs cycle at hypoxia. Front Endocrinol (Lausanne) 2018;9:637. doi: 10.3389/fendo.2018.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiaschi T., Marini A., Giannoni E. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72:5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 57.Apicella M., Giannoni E., Fiore S. Increased lactate secretion by cancer cells sustains non-cell-autonomous adaptive resistance to MET and EGFR targeted therapies. Cell Metab. 2018;28:848–865. doi: 10.1016/j.cmet.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Allen E., Miéville P., Warren C.M. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 2016;15:1144–1160. doi: 10.1016/j.celrep.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C., Liu Z., Bunker E. Sorafenib targets the mitochondrial electron transport chain complexes and ATP synthase to activate the PINK1–Parkin pathway and modulate cellular drug response. J Biol Chem. 2017;292:15105–15120. doi: 10.1074/jbc.M117.783175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen Y.C., Ou D.L., Hsu C. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer. 2013;108:72–81. doi: 10.1038/bjc.2012.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao J., Liu P.P., Hou G. Regulation of stem-like cancer cells by glutamine through beta-catenin pathway mediated by redox signaling. Mol Cancer. 2017;16:51. doi: 10.1186/s12943-017-0623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu R., Li Y., Tian L. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019;443:34–46. doi: 10.1016/j.canlet.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 63.Sohn B.H., Park I.Y., Shin J.H., Yim S.Y., Lee J.S. Glutamine synthetase mediates sorafenib sensitivity in beta-catenin-active hepatocellular carcinoma cells. Exp Mol Med. 2018;50:e421. doi: 10.1038/emm.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun X., Ou Z., Chen R. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun X., Niu X., Chen R. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu I.M., Lai R.K., Lin S.H. Transketolase counteracts oxidative stress to drive cancer development. Proc Natl Acad Sci U S A. 2016;113:E725–E734. doi: 10.1073/pnas.1508779113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee D., Xu I.M.-J., Chiu D.K.-C. Folate cycle enzyme MTHFD1L confers metabolic advantages in hepatocellular carcinoma. J Clin Invest. 2017;127:1856–1872. doi: 10.1172/JCI90253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee D., Xu I.M.-J., Chiu D.K.-C. Induction of oxidative stress through inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology. 2019;69:1768–1786. doi: 10.1002/hep.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao L., Wang X., Tang Y., Huang S., Hu C.A., Teng Y. FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib. J Exp Clin Cancer Res. 2017;36:8. doi: 10.1186/s13046-016-0478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teng Y., Zhao H., Gao L., Zhang W., Shull A.Y., Shay C. FGF19 Protects hepatocellular carcinoma cells against endoplasmic reticulum stress via activation of FGFR4-GSK3beta-Nrf2 signaling. Cancer Res. 2017;77:6215–6225. doi: 10.1158/0008-5472.CAN-17-2039. [DOI] [PubMed] [Google Scholar]
- 71.Masuda M., Chen W.-Y., Miyanaga A. Alternative mammalian target of rapamycin (mTOR) signal activation in sorafenib-resistant hepatocellular carcinoma cells revealed by array-based pathway profiling. Mol Amp; Cell Proteom. 2014;13:1429–1438. doi: 10.1074/mcp.M113.033845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prieto-Dominguez N., Mendez-Blanco C., Carbajo-Pescador S. Melatonin enhances sorafenib actions in human hepatocarcinoma cells by inhibiting mTORC1/p70S6K/HIF-1alpha and hypoxia-mediated mitophagy. Oncotarget. 2017;8:91402–91414. doi: 10.18632/oncotarget.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong X., Song R., Song H. PTEN antagonises Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to hepatocarcinogenesis. Gut. 2014;63:1635–1647. doi: 10.1136/gutjnl-2013-305302. [DOI] [PubMed] [Google Scholar]
- 74.Sounni Nor E., Cimino J., Blacher S. Blocking lipid synthesis overcomes tumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell Metab. 2014;20:280–294. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 75.Lally J.S.V., Ghoshal S., DePeralta D.K. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019;29:174–182.e5. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma M.K.F., Lau E.Y.T., Leung D.H.W. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J Hepatol. 2017;67:979–990. doi: 10.1016/j.jhep.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 77.Martinez-Quetglas I., Pinyol R., Dauch D. IGF2 is up-regulated by epigenetic mechanisms in hepatocellular carcinomas and is an actionable oncogene product in experimental models. Gastroenterology. 2016;151:1192–1205. doi: 10.1053/j.gastro.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Gailhouste L., Liew L.C., Yasukawa K. Differentiation therapy by epigenetic reconditioning exerts antitumor effects on liver cancer cells. Mol Ther. 2018;26:1840–1854. doi: 10.1016/j.ymthe.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lachenmayer A., Toffanin S., Cabellos L. Combination therapy for hepatocellular carcinoma: additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J Hepatol. 2012;56:1343–1350. doi: 10.1016/j.jhep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu M., Zhu W.-W., Wang X. ACOT12-dependent alteration of acetyl-CoA drives hepatocellular carcinoma metastasis by epigenetic induction of epithelial-mesenchymal transition. Cell Metab. 2019;29:886–900.e5. doi: 10.1016/j.cmet.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 81.Zhang D., Tang Z., Huang H. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang L., Zeng J., Geng P. Global metabolic profiling identifies a pivotal role of proline and hydroxyproline metabolism in supporting hypoxic response in hepatocellular carcinoma. Clin Cancer Res. 2018;24:474–485. doi: 10.1158/1078-0432.CCR-17-1707. [DOI] [PubMed] [Google Scholar]
- 83.Dietrich P., Koch A., Fritz V., Hartmann A., Bosserhoff A.K., Hellerbrand C. Wild type Kirsten rat sarcoma is a novel microRNA-622-regulated therapeutic target for hepatocellular carcinoma and contributes to sorafenib resistance. Gut. 2018;67:1328–1341. doi: 10.1136/gutjnl-2017-315402. [DOI] [PubMed] [Google Scholar]
- 84.Xu J., Lin H., Li G. The miR-367-3p increases sorafenib chemotherapy efficacy to suppress hepatocellular carcinoma metastasis through altering the androgen receptor signals. EBioMedicine. 2016;12:55–67. doi: 10.1016/j.ebiom.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang P.-F., Wang F., Wu J. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma. J Cell Physiol. 2019;234:2788–2794. doi: 10.1002/jcp.27095. [DOI] [PubMed] [Google Scholar]
- 86.Wei Y., Chen X., Liang C. A noncoding regulatory RNAs network driven by Circ-CDYL acts specifically in the early stages hepatocellular carcinoma. Hepatology. 2019;0 doi: 10.1002/hep.30795. [DOI] [PubMed] [Google Scholar]
- 87.Li Q., Pan X., Zhu D., Deng Z., Jiang R., Wang X. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR-338-3p/PKM2 axis under hypoxic stress. Hepatology. 2019;70:1298–1316. doi: 10.1002/hep.30671. [DOI] [PubMed] [Google Scholar]
- 88.Ma M., Xu H., Liu G. Metabolism-induced tumor activator 1 (MITA1), an Energy Stress–Inducible Long Noncoding RNA, promotes hepatocellular carcinoma metastasis. Hepatology. 2019;70:215–230. doi: 10.1002/hep.30602. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y., Zhu P., Luo J. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. EMBO J. 2019;38 doi: 10.15252/embj.2018101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng L., Jiang B., Yuan X. Super-Enhancer-Associated Long Noncoding RNA HCCL5 Is Activated by ZEB1 and Promotes the Malignancy of Hepatocellular Carcinoma. Cancer Res. 2019;79:572–584. doi: 10.1158/0008-5472.CAN-18-0367. [DOI] [PubMed] [Google Scholar]