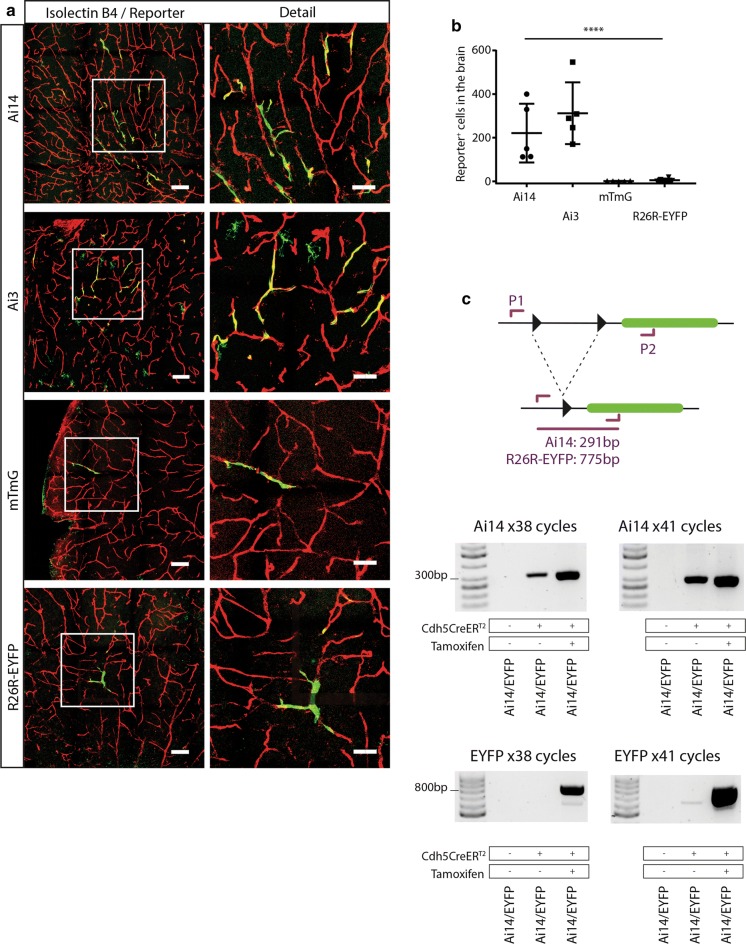
Fig. 5.
Activation of fluorescent reporters under basal CreERT2 activity levels in the brain. a Left column panels: representative images of brains sections from Cdh5(PAC)-CreERT2 mice with either the Ai14, Ai3, mTmG, or R26R-EYFP reporter. Positive endothelial cells can be seen in all brains to varying degrees. Scale bars indicate 100 μm. Right column panels: detailed images of the areas marked with squares in the left panels. Scale bars indicate 50 μm. b Quantification of fluorescent cells in brain sections of the different reporter mice (Ai14: n = 5, Ai3: n = 5, mTmG: n = 5, R26R-EYFP: n = 7). One way ANOVA was performed for statistical analysis. c PCR analysis of Cre-mediated recombination in the Ai14 and R26R-EYFP reporter. Two sets of PCR primers (P1 and P2) were designed to flank the loxP sites (indicated by black arrowheads) with P1 priming upstream of the first loxP site and P2 within the fluorescent gene (indicated in green, for Ai14 and R26R-EYFP respectively). PCRs conditions were optimized to amplify the genomic DNA region that remained after recombination had occurred: 291 bp (Ai14) and 775 bp (R26R-EYFP). After 38 cycles, abundant PCR product was obtained for both Ai14 and R26R-EYFP after Tamoxifen induction, while in the absence of Tamoxifen only PCR product from the Ai14 reporter was detected. After 41 cycles, increased amount of PCR product was obtained for both Ai14 and R26R-EYFP after Tamoxifen induction, and in the non-Tamoxifen-treated Ai14 sample, whereas only a very faint band appeared in the non-Tamoxifen-treated R26R-EYFP sample. In the absence of CreERT2, no product is detected in any PCR