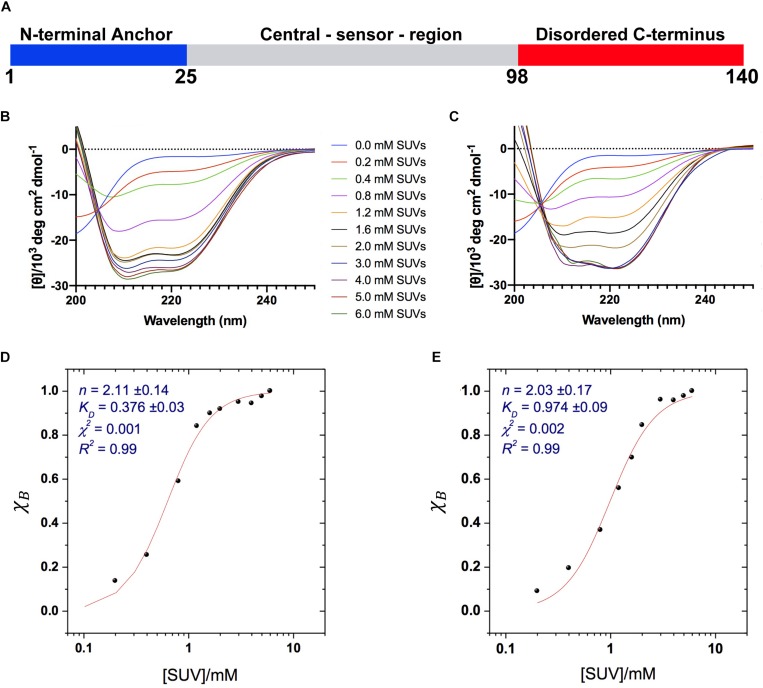
FIGURE 1.
Binding of αS to acidic SUVs. (A) Three regions of αS were found to have specific structural and dynamical properties at the surface of SUV-0% (Fusco et al., 2014). These include the N-terminal anchor (residues 1–25, blue), whose ssNMR resonances spanning the region 6–25 were previously assigned (Fusco et al., 2014), the central sensor region (residues 26–97, gray), and the C-terminal domain (residues 98–140, red) remaining essentially unbound and disordered at the membrane surface. (B–E) CD measurements of αS binding to acidic SUV-0% (B,D) and SUV-31% (C,E). In all measurements the concentration of αS was kept constant at 10 μM whereas the concentrations of SUV-0% and SUV-31% were calculated by considering exclusively the DOPE:DOPS:DOPC component in both types of vesicles. (B,C) CD titration in 20 mM buffer, pH 6.0 measured at 10°C for SUV-0% (B) and SUV-31% (C). (D,E) Fitting of the CD titrations following the signal at 222 nm, [θ]222 nm for SUV-0% (D) and SUV-31% (E). The Hill equation was used to account for both binding constant KD and the cooperativity, which is fitted with the Hill coefficient n.