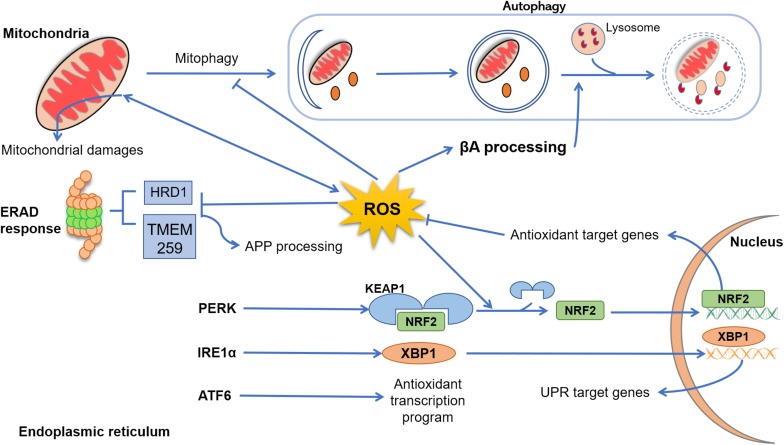
FIGURE 1.
Cross talk between reactive oxygen species (ROS), endoplasmic reticulum (ER), mitochondria, and autophagy. In Alzheimer’s disease (AD), mitochondrial dysfunction progress due to aging, genetic abnormalities, environmental damage, or neuroinflammation, resulting in excessive production of ROS and accumulation of lipids’, proteins’, and nucleic acids’ damage. In physiological conditions, dysfunctional mitochondria are removed from cytoplasm through autophagy (mitophagy), an event that in AD disease is suppressed by excessive levels of ROS and β-amyloid peptide (Aβ). Emerging evidence indicated that ROS elevation may also enhance Aβ processing, inhibiting the fusion between late endosomes and lysosomes. Under several physiological and pathological conditions, the process of protein folding and post-translational modification could be overwhelmed, leading to abnormal levels of ER stress and, collectively, results in the activation of the unfolded protein response (UPR) through three sensor proteins: protein kinase RNA-like ER kinase (PERK), ATF6, and IRE (inositol-requiring protein) 1α. PERK activation leads to a conformational change that results in the release of Nrf2. This release is enhanced by ROS-induced changes, and Nrf2 translocates to the nucleus, where it induces the expression of genes coding antioxidant proteins. Upon stimulation by ER stress, IRE1α processes X-box–binding protein (Xbp1) transcripts, resulting in a splice variant of Xbp1 mRNA (Xbp1s) that encodes the transcription factor XBP1s. Finally, XBP1s translocates to the nucleus and induces an array of genes involved in the recovery of ER functions. After activation, ATF6 translocates to the Golgi, where it is cleaved by two proteases and its cytoplasmic domain is translocated to the nucleus, turning up an antioxidant program. Clearance of misfolded and damaged proteins could be also solved by ER-associated degradation. However, exacerbated ROS levels inhibit the activity of proteins such as HRD1 and TMEM259, resulting in an enhancement of amyloid precursor protein (APP) accumulation and Aβ production.