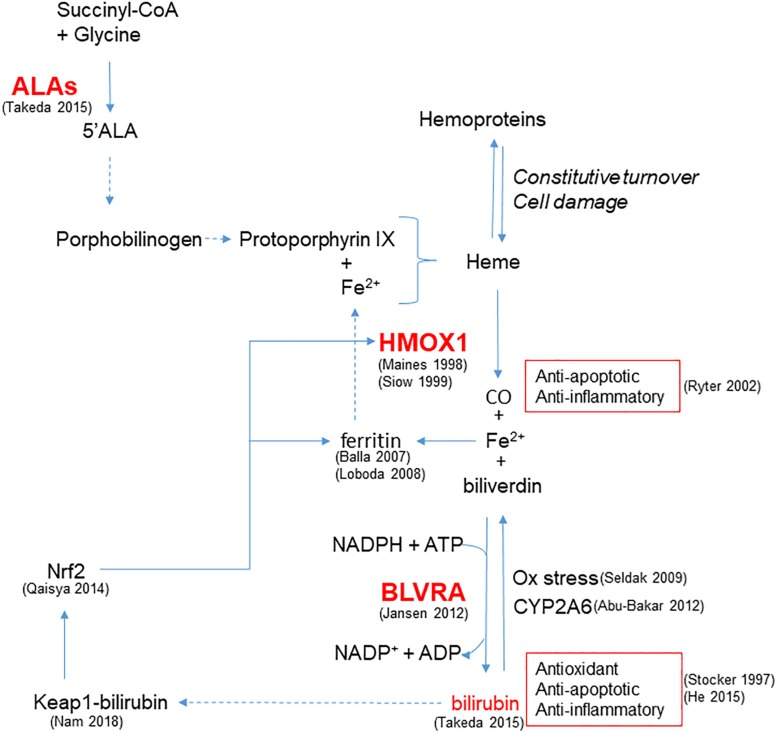
FIGURE 1.
Enzymatic reactions modulated to increase bilirubin generation and cytoprotection. HMOX1 catalyzes the degradation of heme groups to CO, Fe2+ and biliverdin, the latter subsequently converted to bilirubin by BLVRA. By reaction with oxidant species, bilirubin is oxidized back to biliverdin, amplifying the antioxidant effect. Bilirubin is also the substrate of CYP2A6 responsible for its oxidation to biliverdin. Bilirubin and CO exert anti-apoptotic and anti-inflammatory activity. Fe2+ is quenched by the heavy chain of ferritin, and further released to form heme. In addition to iron availability, the synthesis of heme groups depends on the activity of ALAs that catalyzes the reaction between succinyl-CoA and glycine to form 5’ALA; this is then converted to porphobilinogen and protoporphyrin IX that forms heme. Heme groups can also derived from the constitutive turnover of hemoproteins that can be amplified by cell damage. A positive feedback of cytoprotection can be generated by the ability of bilirubin to bind nucleophiles such as thiol reactive cysteines on Keap1, favoring Nrf2-dependent HMOX1 gene transcription. References in brackets.