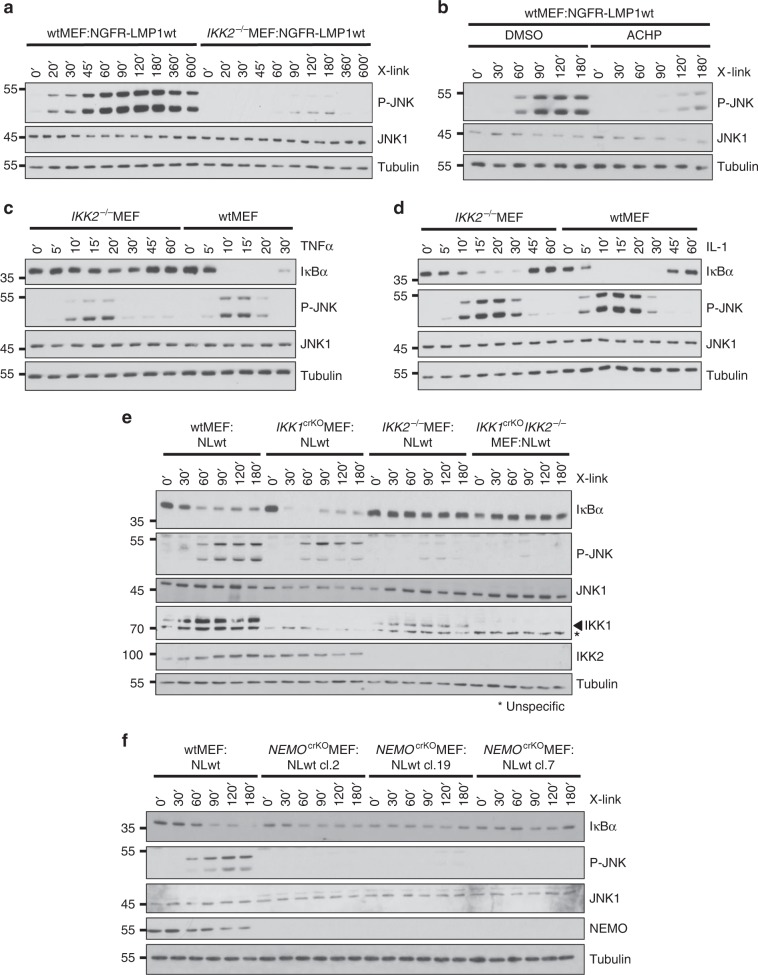
Fig. 2. Essential functions of IKK2 and NEMO in JNK activation by LMP1.
a LMP1 fails to induce JNK after the knockout of IKK2 in MEFs. LMP1 activity was induced by antibody crosslinking and JNK activation was monitored by immunoblotting. b Pharmacological inhibition of IKK activity interferes with JNK activation by LMP1. NGFR-LMP1 was induced in the presence of solvent (DMSO) or 5 μM of the IKK inhibitor VIII (ACHP). c, d IKK2 has a unique role in LMP1 signaling as compared to the TNFα and IL-1 pathways. Wildtype and IKK2−/−MEFs were stimulated with 20 ng/ml TNFα (c) or 10 ng/ml IL-1 (d) for the indicated times. e IKK1 is dispensable for JNK activation by LMP1. IKK1 was targeted by CRISPR/Cas9 in wildtype MEF:NGFR-LMP1wt and IKK2−/−MEF:NGFR-LMP1wt cells. LMP1 activity was induced in non-targeted, IKK1crKOMEF:NGFR-LMP1wt (clone 69) and IKK1crKOIKK2−/−MEF:NGFR-LMP1wt (clone 8) cells. Activation of JNK and canonical NF-κB was monitored. f The knockout of NEMO causes a defect in JNK activation by LMP1. NEMO was inactivated in wtMEF:NGFR-LMP1wt cells by CRISPR/Cas9 technology. NGFR-LMP1 activity was induced in non-targeted cells and the NEMOcrKO clones 2, 7, and 19. JNK and canonical NF-κB were analysed. CRISPR/Cas9 knockouts were verified by immunoblotting (this Figure) and sequencing (see Supplementary Table 2). a–f The data are representative of at least two independent experiments. For immunoblot quantification and statistics see Supplementary Table 3.