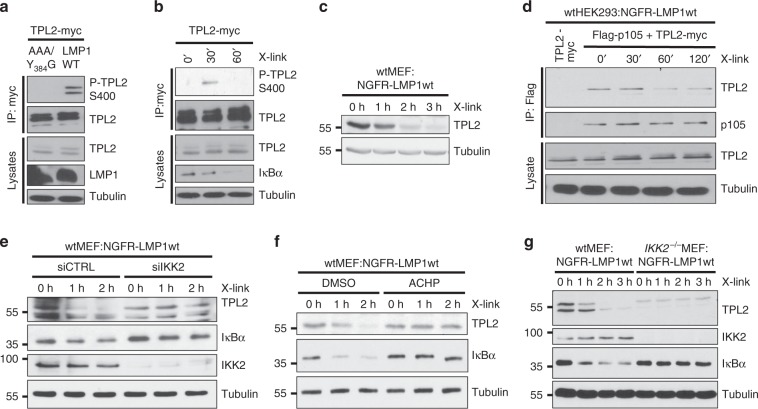
Fig. 4. LMP1 activates TPL2 via IKK2.
a, b LMP1 activity induces TPL2 phosphorylation at serine 400. a HEK293 cells were transfected with TPL2-myc together with HA-LMP1 wildtype or inactive AAA/Y384G. Phosphorylation of immunoprecipitated TPL2-myc was detected with the P-TPL2 S400 antibody. b HEK293 cells, which stably express NGFR-LMP1, were transfected with Myc-tagged TPL2. NGFR-LMP1 activity was induced by antibody crosslinking 24 h after transfection. TPL2-myc was immunoprecipitated and TPL2-myc phosphorylation was detected. c LMP1 activity causes TPL2 degradation. Immunoblot analysis of wtMEF:NGFR-LMP1wt cells induced by antibody crosslinking. d TPL2 is released from its complex with p105 after LMP1 induction. HEK293:NGFR-LMP1wt cells were transfected with Flag-p105 and TPL2-myc vectors. After 24 h, NGFR-LMP1 was induced for the indicated times and Flag-p105 was precipitated via its Flag-tag. Interaction of TPL2 and p105 was analysed using the indicated antibodies. e The knockdown of IKK2 blocks LMP1-induced TPL2 degradation. Cells were transfected twice with IKK2 siRNA (siIKK2) or non-targeting control siRNA (siCTRL). After 24 h, NGFR-LMP1 activity was induced and TPL2, IκBα, and IKK2 levels were analysed. f LMP1-induced TPL2 degradation depends on IKK activity. NGFR-LMP1 was induced in MEFs by antibody crosslinking in the presence of DMSO or 5 µM ACHP. g LMP1 fails to induce TPL2 degradation in the absence of IKK2. NGFR-LMP1 activity was induced in wildtype and IKK2−/− MEFs, and cellular protein levels were analysed. b This experiment confirms the results of a and was performed once. a, c–g The data are representative of at least two independent experiments. For immunoblot quantification and statistics see Supplementary Table 3.