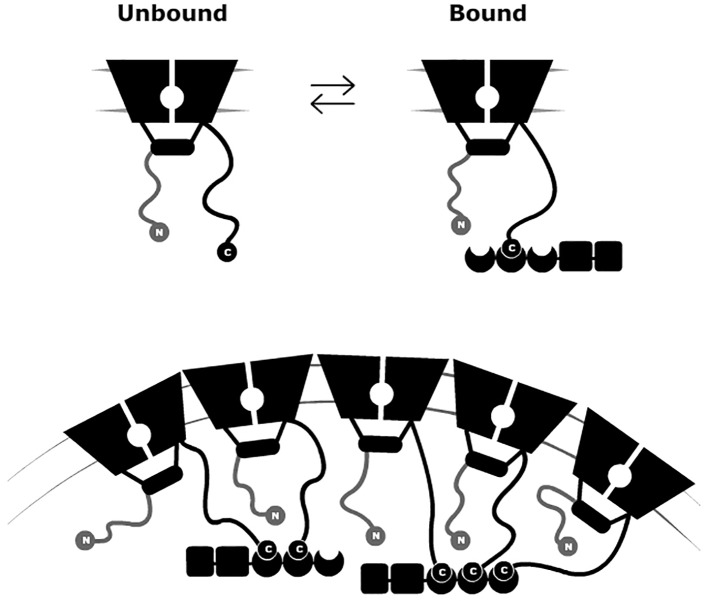
Figure 2.
A “ball and chain” mechanism for Kv channel clustering. Schematic representation of the “ball and chain” mechanism for channel binding to PSD-95 (upper panel). In the inter-molecular ‘ball and chain’ binding mechanism, the interaction of the Kv channel with the membrane-associated PSD-95 scaffold protein is precisely timed, as determined by C-terminal chain length, upon binding of the “chain”-tethered peptide “ball” to the PSD-95 PDZ domain(s). Given the stoichiometry of the interaction and the ability of PSD-95 to aggregate, channel clustering results (lower panel). The membrane-embedded portion corresponds to the channel voltage-sensor and pore domains, while the rectangular shape corresponds to the T1 assembly domain. The crescent, box, and rectangular shapes represent the PDZ, SH3, and guanylate kinase-like domains of the PSD-95 protein, respectively. PSD, post-synaptic density.