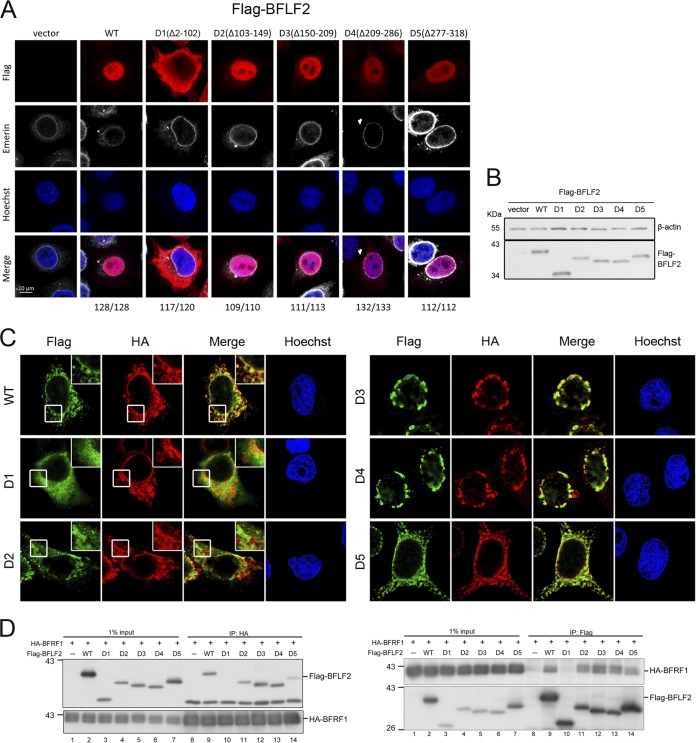
FIG 2.
The region aa 2 to 102 of BFLF2 is required for nuclear targeting and interaction with BFRF1. (A) Plasmids expressing Flag-BFLF2 wild type, F2D1, F2D2, F2D3, F2D4, or F2D5 were transfected into slide-cultured HeLa cells. At 24 h posttransfection (hpt), cells were fixed with 4% paraformaldehyde, immunostained for Flag (red) and emerin (white), stained with Hoechst 33258 to indicate cellular DNA (blue), and analyzed by confocal microscopy. The cell numbers showing the representative pattern are indicated at the bottom. (B) At the same time, a portion of cell lysates was analyzed by immunoblotting against Flag and β-actin. The experiments shown in panels A and B were performed 3 times. Representative data from three independent experiments are shown. (C) Plasmids expressing HA-BFRF1 together with Flag-BFLF2 wild type, mutant F2D1 (Δ2-102), F2D2 (Δ103-149), F2D3 (Δ150-209), F2D4 (Δ209-286), or F2D5 (Δ277-318) were transfected into slide-cultured HeLa cells. At 24 hpt, the cells were fixed, immunostained for Flag (green) and HA (red), stained with Hoechst 33258 to indicate cellular DNA (blue), and analyzed by confocal microscopy. Cells showing representative staining patterns are displayed. The insets of Flag-WT, Flag-F2D1, and Flag-F2D2 are the enlarged images to show different cytoplasmic staining patterns. (D) The HA-BFRF1 plasmid was transfected with Flag-BFLF2 wild type, F2D1, F2D2, F2D3, F2D4, or F2D5 into HeLa cells. The lysates were immunoprecipitated with antibody against Flag and HA. The immune complexes were then resolved by SDS-PAGE and immunoblotted with antibodies against Flag or HA. Representative data from two independent experiments are shown.