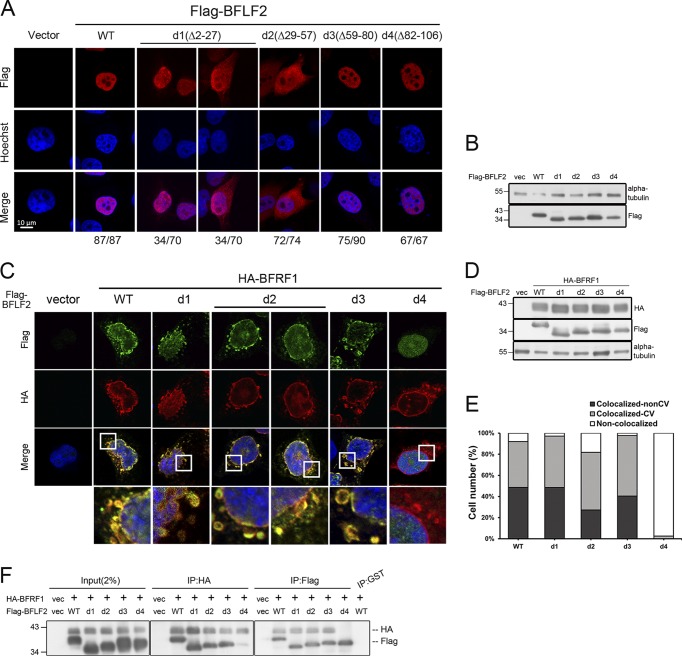
FIG 3.
Amino acids 29 to 57 of BFLF2 are required for nuclear targeting and aa 81 to 107 of BFLF2 are required for BFRF1 interaction. (A) Plasmid pcDNA3.0 or plasmid expressing Flag-BFLF2 wild type, F2d1, F2d2, F2d3, and F2d4 were transfected individually into slide-cultured HeLa cells. At 24 hpt, cells were fixed and stained for Flag (red) and cellular DNA (blue). The images were analyzed by confocal microscopy. The cells with intranuclear or cytoplasmic distribution of BFLF2 were counted. The representative patterns are displayed. (B) Lysates from cells shown in panel A were harvested and analyzed by immunoblotting with antibodies against Flag and α-tubulin, which served as a loading control. (C) Plasmid expressing HA-BFRF1 was transfected with Flag-BFLF2 wild type or serial small-deletion mutants into slide-cultured HeLa cells. At 24 hpt, slides were fixed, stained with antibody against Flag (green) and HA (red), stained with Hoechst indicating cellular DNA (blue), and analyzed by confocal microscopy. (D) Lysates from cells shown in panel C were analyzed by immunoblotting against Flag, HA, and α-tubulin. Both experiments shown in panels C and D were performed two times, and representative data are shown. (E) Bar graph shows the percentages of cells with subcellular colocalization of BFRF1 and BFLF2 at the nuclear envelope or within cytoplasmic vesicles (CV; n = 22 to 42) from the experiment shown in panel C. (F) Plasmid expressing HA-BFRF1 was transfected with Flag-BFLF2 wild type or serial deletion mutants into HeLa cells. At 24 hpt, the lysates were harvested and immunoprecipitated with antibody against HA (top) or Flag (bottom). The immune complexes were then resolved by 10% SDS-PAGE and immunoblotted with antibodies against HA or Flag. The experiment was performed three times, and representative data from three independent experiments are shown.