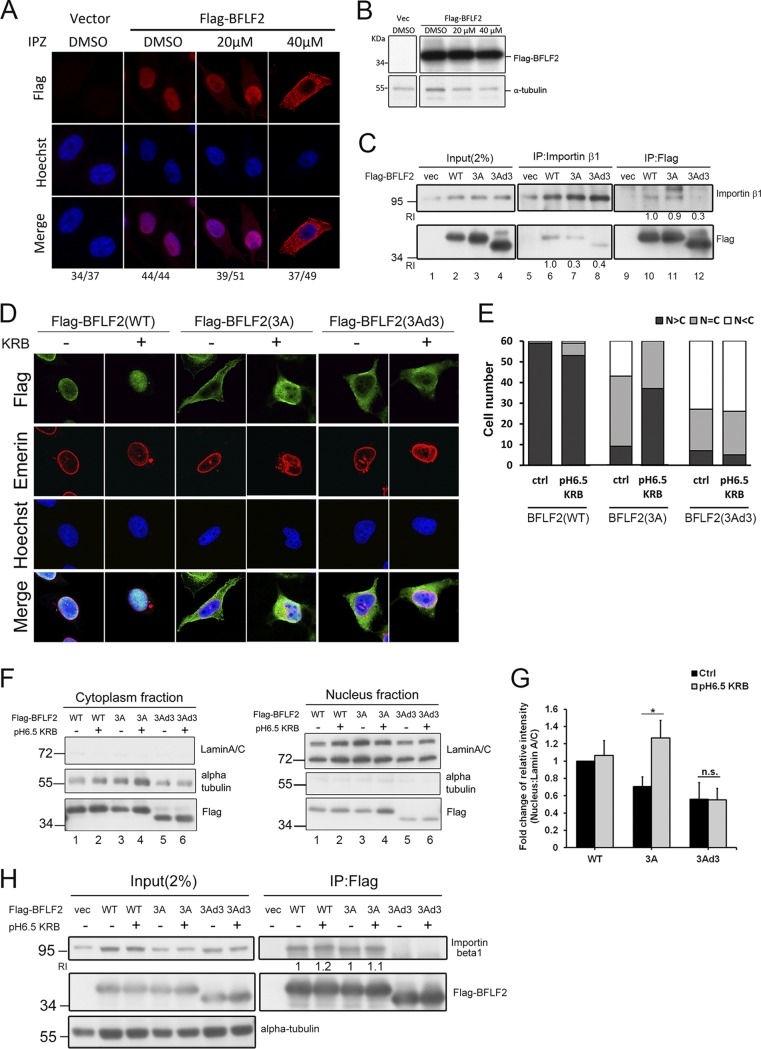
FIG 6.
The nuclear localization of BFLF2 is importin β1-dependent, and low-pH KRB buffer solution enhances the nuclear accumulation of BFLF2. (A) Slide-cultured HeLa cells were transfected with vector or Flag-BFLF2. At 6 h posttransfection, IPZ (20 μM, 40 μM) or DMSO was added to the medium. After incubation for another 24 h, the cells were fixed and stained for Flag (red), and cellular DNA was stained with Hoechst 33258 (blue). The cells were analyzed by confocal microscopy. The experiment was performed two times, and representative data are shown. Cells showing representative staining patterns are displayed. (B) Lysates were harvested from cells used for panel A and analyzed by immunoblotting against Flag and α-tubulin. (C) HeLa cells were transfected with Flag-BFLF2, Flag-F2(3A), or Flag-F2(3Ad3), and the lysates were harvested for reciprocal coimmunoprecipitation with Flag or importin β antibodies to indicate the interaction between BFLF2 protein and importin β. The immunocomplexes were then resolved by SDS-PAGE and immunoblotted with antibodies against Flag and importin β1. The experiment was performed two times, and representative data are shown. Relative intensities (RI) of immunoprecipitated Flag-F2(3A) or Flag-F2(3Ad3) adjusted by the protein levels of immunoprecipitated importin β (lanes 7 and 8) were compared to that of Flag-BFLF2(WT) (lane 6). Similar quantitations were performed for coimmunoprecipitation with Flag antibody (lanes 10 to 12). (D) Slide-cultured HeLa cells were transfected with Flag-BFLF2, F2(3A), or F2(3Ad3). At 24 hpt, pH 6.5 KRB buffer or fresh medium was added to the dish. After incubation for 1 h, the cells were fixed and stained for Flag (green), emerin (red), and cellular DNA (with Hoechst 33258; blue). The cells were analyzed by confocal microscopy. Cells showing representative staining patterns are displayed. (E) Bar graph shows the percentages of cells displaying indicated subcellular distribution of BFLF2 (n = 60). (F) Immunoblotting of the lysates from cells used for data shown in panel D was performed using anti-Flag, anti-lamin A/C, or anti-α-tubulin antibodies after subcellular fractionation for cytoplasmic (left) and nuclear (right) fractions. The experiment was performed three times, and representative data are shown. (G) Bar graph shows the fold changes in relative intensities of Flag-BFLF2 compared to that of lamin A/C in the nuclear fraction with non-KRB treatment (Ctrl). Error bars were calculated with data from 3 independent experiments. The statistically significant differences were calculated by the paired Student's t test and are indicated at the top. *, P < 0.05: n.s., no significant differences. (H) Lysates from cells with the same setting as those in panel D were harvested and immunoprecipitated with antibody against importin β1. The immunocomplexes were then resolved by SDS-PAGE and immunoblotted with antibodies against Flag and importin β1. RI indicates the relative intensities of immunoprecipitated importin β adjusted by the protein levels of immunoprecipitated Flag-F2(3A) and compared to that of Flag-BFLF2(WT), which was not treated with pH 6.5 KRB buffer. Similar quantitations were performed for coimmunoprecipitation with Flag antibody.