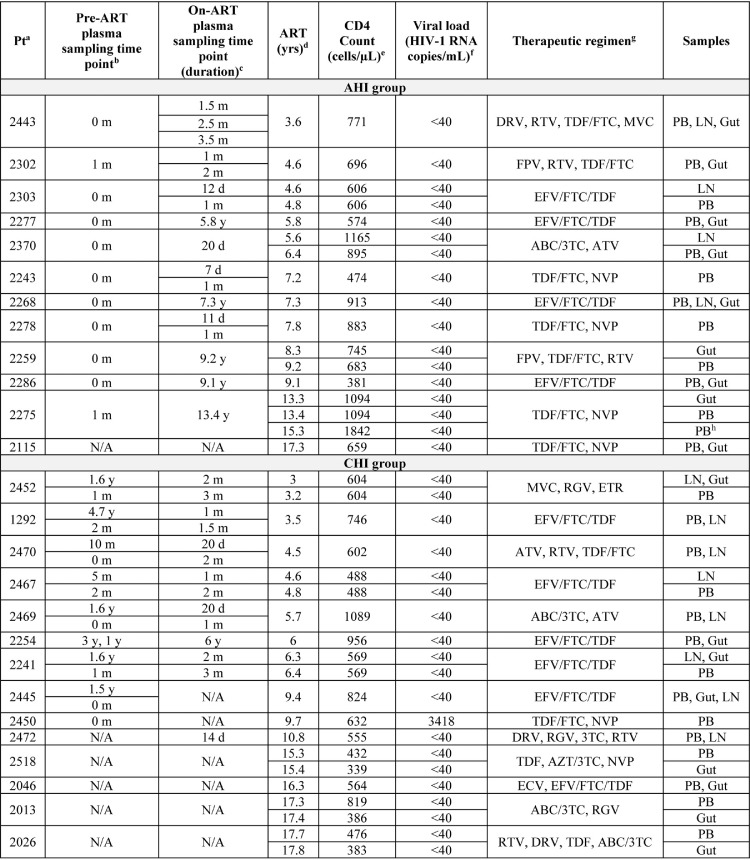
TABLE 1.
Participant demographics and clinical samples
Pt, participant.
Years (y) and months (m) before ART initiation. Only the CHI group was used for analysis. NA, not applicable.
Years, months, or days (d) after ART initiation. Only the CHI group was used for analysis. NA, not applicable.
Duration on ART at the time of sample isolation.
CD4 cell count at the time of sample isolation.
Viral load at the time of sample isolation.
Therapeutic regimen at the time of sample isolation. 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; AZT, zidovudine; DRV, darunavir; ECV, entecavir; EFV, efavirenz; ETR, etravirine; FPB, fosamprenavir; FTC, emtricitabine; MVC, maraviroc; NVP, nevirapine; RGV, raltegravir; RTV, ritonavir; TDF, tenofovir.
Excluded from analyses of the fold effect of the proportion of cells infected for cross-sectional analysis.