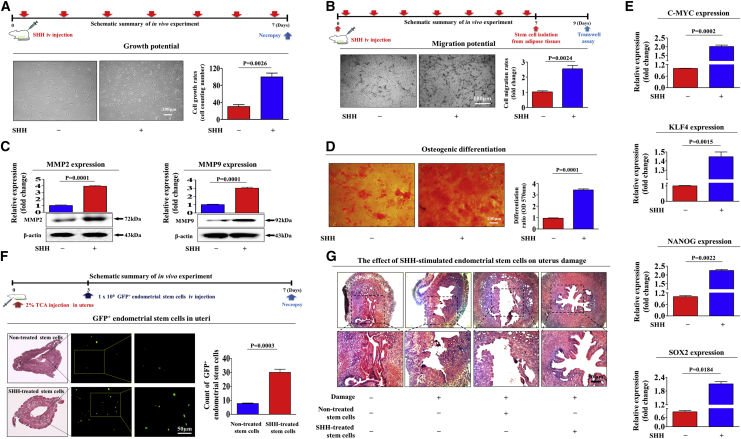
Figure 7.
SHH Stimulates the Therapeutic Potential of Endometrial Stem Cells by Promoting Migratory and Differentiation Capacities In Vivo in an Endometrial Ablation Animal Model
Mice were treated daily for 7 days with SHH (1 mg/kg, intravenously) or vehicle (PBS). Stem cells were isolated from mouse adipose tissue, and the changes in cell viability were analyzed by an MTT assay (A). The in vivo effects of SHH on the migratory capacity of the stem cells were analyzed by a Transwell assay (B) and western blotting for MMP-2 and MMP-9 (C). The changes in osteoblast differentiation were analyzed by alizarin red staining. The relative quantification of calcium mineral content was performed by measuring the absorbance at a wavelength of 570 nm (D). The real-time PCR results showed changes in the expression of the mouse stem cell markers C-MYC, KLF4, NANOG, and SOX2 after SHH treatment in vivo (E). The 2% TCA treatment (150 μL, administered directly into the uterine horn) caused significant histological uterine endometrial ablation compared with the vehicle (PBS) control. SHH-stimulated or nonstimulated endometrial stem cells (1 × 106 cells) were labeled with GFP and injected intravenously into the tail veins of 7-week-old immunodeficient NSG mice with acute TCA-induced endometrial ablation. The mice were sacrificed 7 days after the GFP-labeled endometrial stem cells were injected. Green fluorescent images of consecutive sections showed the presence of GFP-labeled cells (F). Uterine endometrial tissue was collected and subjected to hematoxylin and eosin (H&E) staining. TCA-induced loss of the endometrial functional layer with degenerative changes was significantly more alleviated by the transplantation of SHH prestimulated endometrial stem cells (G). β-Actin was used as the internal control. The data represent the mean ± SEM from eight independent experiments.