Abstract
Background
Although regorafenib (REG) and trifluridine/tipiracil (FTD/TPI) have been recognised as standard treatments in metastatic colorectal cancer (mCRC), the best option remains unclear. Pretreatment tumour growth rate (TGR) is associated with radiotherapeutic efficacy in laryngeal cancer. However, no reports are available on the association between TGR during preceding treatment and the efficacy of REG or FTD/TPI.
Patients and methods
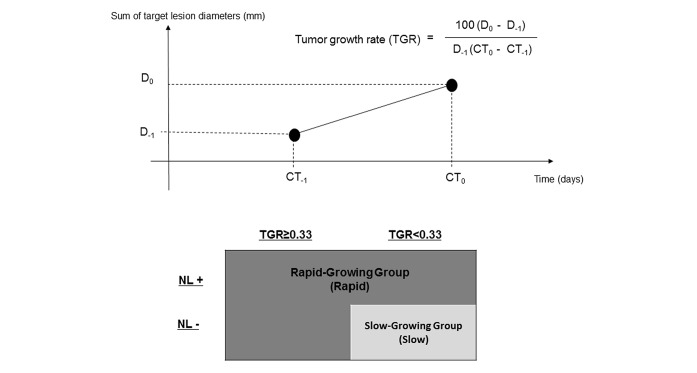
We retrospectively analysed the data of consecutive mCRC patients treated with REG or FTD/TPI and classified them into slow-growing or rapid-growing (SG or RG) groups according to TGR and emergence of new lesion (NL+) or their absence (NL−) during preceding treatment period [SG: NL− with low TGR (<0.33%/day); RG: NL+ or high TGR (≥0.33%/day)].
Results
A total of 244 patients (RG/SG, 133/111; REG/FTD/TPI, 132/112) were eligible. The RG proportion with a long duration from first-line chemotherapy and the SG proportion with elevated alkaline phosphatase were higher in REG, whereas the SG proportion with performance status 2 was higher in FTD/TPI. The disease control rates (DCRs) were similar between REG and FTD/TPI (24%/30%; OR: 0.74; p=0.44; adjusted OR: 0.73; p=0.47) in the RG, whereas the DCR was significantly higher for FTD/TPI than for REG (47%/26%; OR: 2.56; p=0.029; adjusted OR: 3.38; p=0.01) in the SG.
Conclusions
TGR and NL during preceding treatment may be helpful for drug selection in refractory mCRC patients to be treated with REG or FTD/TPI. However, further studies are needed to confirm the value of TGR for drug selection.
Keywords: predictive marker, retrospective study, chemotherapy
Key questions.
What is already known about this subject?
Regorafenib (REG) and trifluridine/tipiracil (FTD/TPI) both prolong the survival of patients with refractory metastatic colorectal cancer. However, which of the two drugs should be administered first remains controversial.
What does this study add?
Based on results of this study, in patients with low tumour growth rate (TGR) without the emergence of new lesion in preceding treatment, FTD/TPI showed favourable disease control rate, which suggested that patients with slow progression are better candidates for FTD/TPI than for REG. However, in patients with high TGR or emergence of new lesion, both drugs showed similar disease control rate; therefore, they are equally recommended for patients with rapid progression in preceding treatment.
How might this impact on clinical practice?
Although predictive biomarkers for the efficacy and safety of REG and FTD/TPI have not been established, our results suggest that TGR and the emergence of new lesion during preceding treatment period may be helpful for drug selection in patients with refractory metastatic colorectal cancer to be treated with REG or FTD/TPI in clinical practice.
Introduction
Regorafenib1 (REG) and trifluridine/tipiracil2 (FTD/TPI) have been recognised as standard treatments in refractory metastatic colorectal cancer (mCRC). Although no head-to-head trials have been reported, because REG and FTD/TPI have similar efficacies and different toxicities, we select one of these active agents considering the toxicity profile, and it is unclear which drug should be administered first in clinical practice.3 4 Because both drugs have limited efficacy without objective tumour response, a predictive marker for response to these drugs is needed.
Recently, it has been reported that the pretreatment tumour growth rate (TGR) was associated with survival in non-small cell lung and laryngeal cancers treated with radiation therapy.5 6 We hypothesised that TGR during preceding treatment would have different effects on the efficacies of REG and FTD/TPI, which have different mechanisms of action and that TGR during preceding treatment period would be helpful for drug selection in patients with mCRC to be treated with REG or FTD/TPI.
Methods
Patients
This was a retrospective study to evaluate the association between TGR during preceding treatment and the efficacy of REG and FTD/TPI at three institutions. We evaluated patients who were treated with REG or FTD/TPI at the Aichi Cancer Center Hospital, Shizuoka Cancer Center and at Hokkaido University Hospital from May 2013 to December 2016. The eligibility criteria were as follows: (1) histologically confirmed unresectable colorectal adenocarcinoma; (2) no prior treatment with REG or FTD/TPI; (3) refractory or intolerant to fluoropyrimidines, oxaliplatin, irinotecan, anti-vascular endothelial growth factor (VEGF) antibodies and anti-epidermal growth factor receptor (EGFR) antibodies (if KRAS exon 2 wild-type tumours); (4) preceding treatment was chemotherapy; (5) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2; (6) measurable lesion according to RECIST version 1.1; (7) adequate bone marrow, hepatic and renal function; and (8) CT was performed at least once during preceding chemotherapy within 30 days before starting REG or FTD/TPI and at least once after starting REG or FTD/TPI. All the patients provided written informed consent for the treatment.
Treatments
REG (160 mg) was administered once daily on days 1–21 with 7 days of rest. FTD/TPI (35 mg/m2) was administered twice daily 5 days a week with 2 days of rest for 2 weeks, followed by a 14-day rest period. Both drugs were repeated every 4 weeks. The treatments were continued until disease progression, death, unacceptable toxicities or the patient’s refusal. We included any patients whose initial dose was reduced because of the patient’s desire or physician’s decision in this study.
Calculation of TGR and method of classification
TGR was calculated as follows:
TGR=100(D0 − D−1)/D−1/(CT0 − CT−1),
where CT0 is the date of CT at progressive disease judged by physicians in preceding treatment, CT−1 is the date of CT directly preceding CT0 and Dn is the sum of target lesion diameters at CTn (according to RECIST version 1.1).
The patients were classified into two groups according to TGR and whether a new lesion (NL) emerged. A cut-off value of TGR was defined as 0.33%/day, which was equal to 20%/2 months or 73%/2 months converted to volume, taking the median TGR (0.32%/day) and clinical significance into account. Emergence of a new lesion (NL; NL+) was defined as an emergence at a new site that did not have metastases when preceding treatment was started. The slow-growing group (slow group) was defined as low TGR (<0.33%/day) and no emergence of NL (NL−), and the rapid-growing group (rapid group) was defined as high TGR (≥0.33%/day) and NL− and NL+ irrespective of TGR (figure 1).
Figure 1.
Definition of TGR and grouping according to TGR and NL+ or NL−. CT0 is the date of CT at progressive disease judged by physicians in preceding treatment, CT−1 is the date of CT directly preceding CT0 and Dn is the sum of target lesion diameters at CTn. The slow-growing group was defined as low TGR (<0.33%/day) and NL−, and the rapid-growing group was defined as high TGR (≥0.33%/day) and NL− and NL+ irrespective of TGR. TGR, tumour growth rate; NL+, emergence of new lesion; NL−, absence of new lesion.
We varied the cut-off values of TGR because it was unclear whether the cut-off value of TGR in the present study was appropriate or not.
Evaluation of treatment and statistical analysis
All the patients underwent CT at least once after starting REG or FTD/TPI. The efficacy of REG and FTD/TPI were evaluated by disease control defined as a complete response, partial response or stable disease according to RECIST version 1.1.
The differences in the patient characteristics and disease control rates (DCRs) between REG and FTD/TPI were compared by using Fisher’s exact test with OR and 95% CI based on logistic regression analysis. The differences in DCR were also evaluated by multivariate analyses using stepwise logistic regression and presented as adjusted ORs. In the multivariate analyses for DCR, the following variables were included: age (<65 vs ≥65 years); sex; ECOG PS (0–1 vs 2); histological type (well or moderate vs poor or mucinous); site of primary tumour (right-sided cancer vs left-sided cancer; right-sided cancer: caecum, ascending colon and transverse colon; left-sided cancer: descending colon, sigmoid colon and rectum); resection of primary tumour (yes vs no); number of metastatic sites (1–2 vs ≥3); liver metastases (yes vs no); peritoneal metastases (yes vs no); KRAS exon 2 status (wild-type vs mutant); time from initiation of first-line chemotherapy (<18 months vs ≥18 months); white blood cell (WBC) count (<10 000 vs ≥10 000/µL); alkaline phosphatase (ALP) levels (<300 vs ≥300 IU/L); and lactate dehydrogenase (LDH) levels (<400 vs ≥400 IU/L). Progression-free survival (PFS) was defined as the time from the first administration of treatment to the first radiological or clinical observation of disease progression or death due to any cause, whichever occurred first. Overall survival (OS) was defined as the time from the first treatment until death from any cause, with surviving patients censored up to the last follow-up date. The median PFS and OS were estimated using the Kaplan-Meier method. HR and 95% CI were estimated using the Cox proportional hazards model. Adjusted HRs for PFS and OS were calculated by applying a multivariate Cox proportional hazard model based on the parameters with p values of <0.1 in the univariate analysis.
JMP version 10 (SAS Institute) was used to perform all the statistical analyses. All the statistical tests were two sided, with p values of <0.05 considered as indicative of statistical significance.
Results
Patient characteristics
Between May 2013 and December 2016, 388 patients with unresectable mCRC received REG or FTD/TPI for the first time. We excluded 34 patients who had not received fluoropyrimidines, oxaliplatin, irinotecan, anti-VEGF antibodies or anti-EGFR antibodies (if KRAS exon 2 wild-type tumours), seven patients whose preceding treatment was radiotherapy or observation, 23 patients who did not have measurable lesions, 3 patients without CT that was performed at least once during preceding chemotherapy, 33 patients without CT that was performed within 30 days before starting REG or FTD/TPI and 44 patients without CT that was performed at least once after starting REG or FTD/TPI. Therefore, 132 and 112 patients who had received REG (REG group) and FTD/TPI (FTD/TPI group), respectively, were analysed as eligible patients. The rapid group had 133 patients, including 74 patients treated with REG and 59 patients treated with FTD/TPI, and the slow group had 111 patients, including 58 patients treated with REG and 53 patients treated with FTD/TPI (online supplementary figure 1).
esmoopen-2019-000584supp001.pdf (54.9KB, pdf)
In the rapid group, the proportion of patients who had high LDH levels was lower in the FTD/TPI group than in the REG group (20% vs 36%) and that of patients who had <18 months as time from initiation of first-line chemotherapy was higher (46% vs 22%). In the slow group, the proportion of patients who had high ALP levels was lower in the FTD/TPI group than in the REG Group (57% vs 78%) and that of the patients who had ECOG PS 2 was higher (13% vs 2%). Almost all other baseline characteristics were similar between the REG and FTD/TPI groups either in the rapid group or the slow group (table 1).
Table 1.
Patient characteristics
| Characteristics | Rapid group n=133 | Slow group n=111 | ||||
| REG N=74 (%) |
FTD/TPI N=59 (%) |
P value | REG N=58 (%) |
FTD/TPI N=53 (%) |
P value | |
| Age (years) | ||||||
| <65 | 40 (54) | 30 (51) | 0.73 | 29 (50) | 21 (40) | 0.34 |
| ≥65 | 34 (46) | 29 (49) | 29 (50) | 32 (60) | ||
| Sex | ||||||
| Male | 44 (59) | 29 (49) | 0.29 | 33 (57) | 38 (72) | 0.12 |
| Female | 30 (41) | 30 (51) | 25 (43) | 15 (28) | ||
| ECOG performance status | ||||||
| 0–1 | 65 (88) | 54 (92) | 0.58 | 57 (98) | 46 (87) | 0.03 |
| 2 | 9 (12) | 5 (8) | 1 (2) | 7 (13) | ||
| Histological type | ||||||
| Well/moderately | 67 (91) | 55 (93) | 0.75 | 56 (96) | 49 (92) | 0.42 |
| Poorly/mucinous | 7 (9) | 4 (7) | 2 (4) | 4 (8) | ||
| Site of primary tumour | ||||||
| Right-sided colon* | 27 (36) | 14 (24) | 0.18 | 14 (24) | 12 (23) | 1.00 |
| Left-sided colon†/rectum | 47 (64) | 45 (76) | 44 (76) | 41 (77) | ||
| Prior tumour resection | ||||||
| Yes | 64 (86) | 44 (75) | 0.12 | 47 (81) | 39 (74) | 0.37 |
| No | 10 (14) | 15 (25) | 11 (19) | 14 (26) | ||
| Metastatic sites | ||||||
| Liver | 48 (65) | 37 (63) | 0.86 | 32 (55) | 22 (42) | 0.18 |
| Peritoneum | 24 (32) | 20 (34) | 1.00 | 15 (26) | 16 (30) | 0.67 |
| Number of metastatic sites | ||||||
| 1–2 | 48 (65) | 30 (51) | 0.11 | 32 (55) | 34 (64) | 0.44 |
| ≥3 | 26 (35) | 29 (49) | 26 (45) | 19 (36) | ||
| KRAS exon 2 status | ||||||
| Wild-type | 45 (61) | 36 (61) | 1.00 | 28 (48) | 23 (43) | 0.70 |
| Mutant | 29 (39) | 23 (39) | 30 (52) | 30 (57) | ||
| Time from initiation of first-line chemotherapy (months) | ||||||
| <18 | 16 (22) | 27 (46) | 0.0005 | 10 (17) | 13 (25) | 0.36 |
| ≥18 | 58 (78) | 32 (54) | 48 (83) | 40 (75) | ||
| Biologicals in previous chemotherapy | ||||||
| No | 5 (8) | 6 (10) | 0.89 | 6 (10) | 5 (9) | 0.80 |
| Bevacizumab | 40 (54) | 32 (54) | 36 (62) | 36 (68) | ||
| Anti-EGFR agents | 29 (39) | 21 (36) | 16 (28) | 12 (23) | ||
| WBC (/μL) | ||||||
| <10 000 | 66 (89) | 54 (92) | 0.77 | 52 (90) | 49 (92) | 0.74 |
| ≥10 000 | 8 (11) | 5 (8) | 6 (10) | 4 (8) | ||
| ALP (IU/L) | ||||||
| <300 | 18 (24) | 16 (27) | 0.84 | 13 (22) | 23 (43) | 0.03 |
| ≥300 | 56 (76) | 43 (73) | 45 (78) | 30 (57) | ||
| LDH (IU/L) | ||||||
| <400 | 47 (64) | 47 (80) | 0.06 | 42 (72) | 43 (81) | 0.37 |
| ≥400 | 27 (36) | 12 (20) | 16 (28) | 10 (19) | ||
*Caecum, ascending colon and transverse colon.
†Descending colon and sigmoid colon.
ALP, alkaline phosphatase;ECOG, Eastern Cooperative Oncology Group;FTD/TPI, trifluridine/tipiracil; LDH, lactate dehydrogenase; REG, regorafenib; WBC, white blood cell.
Because the CT scans were managed according to the local practice, there were substantial differences between patients in the intervals between the two scans used to assess TGR. To check whether this affected the allocation of patients into the rapid and slow groups, we analysed the numbers allocated to each group with intervals between the scans of ≤6 (rapid group, n=17; slow group, n=10), 6–12 (rapid group, n=83; slow group, n=66) and >12 weeks (rapid group, n=32; slow group, n=36). The results are summarised in online supplementary table 1. The proportions allocated to the rapid and slow groups were similar within each of these range of intervals.
esmoopen-2019-000584supp002.pdf (22KB, pdf)
Comparisons of DCRs and PFS between the REG and FTD/TPI treatment groups within the rapid and slow groups
The interval from the initiation of REG or FTD/TPI to the first CT evaluation was similar for the patients receiving REG and FTD/TPI in the rapid and slow groups (online supplementary table 2).
esmoopen-2019-000584supp003.pdf (22.1KB, pdf)
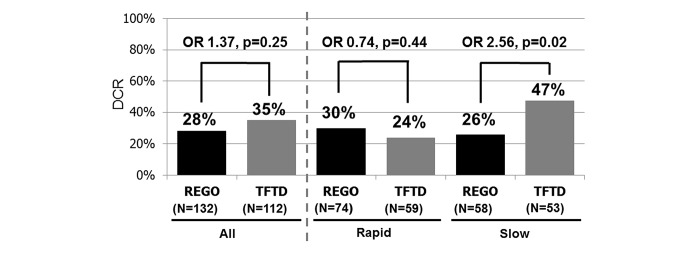
Among the total 244 patients, the DCR in the FTD/TPI group was similar to that in the REG group (35% vs 28%, respectively; OR: 1.37; 95% CI 0.80 to 2.37; p=0.25). All the patients had stable disease or progressive disease. In the rapid group, the DCR in the FTD/TPI group was similar to that in the REG group (24% vs 30%; OR: 0.74; 95% CI, 0.33–1.59; p = 0.44). Conversely, in the Slow Group, the DCR in the FTD/TPI Group was significantly higher than that in the REG Group (47% vs. 26%; OR, 2.56; 95%CI, 1.16–5.78; p = 0.019). The interaction test showed a significant interaction between TGR (Rapid/Slow Group) and treatment (REG/FTD/TPI) (p = 0.027).
In the multivariate analysis using stepwise logistic regression of predictive factors for obtaining disease control, the choice of REG or FTD/TPI was an independent predictive factor for obtaining disease control in the slow group (adjusted OR: 3.38; 95% CI 1.34 to 9.09; p=0.01) with the covariates of sex, histological type, number of metastatic sites, resection of primary tumour and WCC but was not a predictive factor in the rapid group (adjusted OR: 0.73; 95% CI 0.31 to 1.69; p=0.47) with the covariates of ECOG PS, time from initiation of first-line chemotherapy and liver metastases (figure 2).
Figure 2.
DCRs between REG and FTD/TPI groups within the rapid or slow groups. In the rapid group, the DCR in the FTD/TPI group was similar to that in the reg group, whereas in the slow group, the DCR in the FTD/TPI group was significantly higher than that in the reg group. DCR, disease control rate; REG, regorafenib; FTD/TPI, trifluridine/tipiracil.
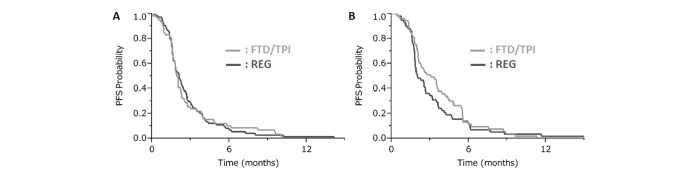
The PFS and OS in the FTD/TPI group were similar to those in the REG group (median PFS: 2.2 vs 2.1 months, respectively; HR: 0.91; 95% CI 0.71 to 1.18; p=0.48; median OS: 7.6 vs 6.8 months, respectively; HR: 0.86; 95% CI 0.66 to 1.13; p=0.30). In the rapid group, the PFS and OS in the FTD/TPI group were similar to those in the REG group (median PFS: 2.0 vs 2.1 months, respectively; adjusted HR: 0.91; 95% CI 0.61 to 1.37; p=0.66; median OS: 6.4 vs 6.7 months, respectively; adjusted HR: 0.97; 95% CI 0.65 to 1.43; p=0.87). In the slow group, the FTD/TPI group showed numerically better PFS and OS values than those for the REG group but without statistical significance (median PFS: 3.1 vs 2.0 months, respectively; adjusted HR: 0.77; 95% CI 0.52 to 1.15; p=0.20; median OS: 8.0 vs 7.0 months, respectively; adjusted HR: 0.79; 95% CI 0.51 to 1.24; p=0.31; figure 3).
Figure 3.
PFSs between the reg and FTD/TPI groups: (A) Rapid group and (B) slow group. In the rapid group, the PFS in the FTD/TPI group was similar to that in the REG group, whereas in the slow group, the PFS in the FTD/TPI group was longer than that in the REG group. PFS, progression-free survival; REG, regorafenib; FTD/TPI, trifluridine/tipiracil.
There was crossover from REG to FTD/TPI or vice versa by a total of 109 patients. The DCR analysis was repeated for this subset of patients. In the rapid group, the FTD/TPI group presented a trend towards lower DCR than that the REG group (16% vs 36%; OR: 0.33; 95% CI 0.08 to 1.13; p=0.08). In the slow group, the DCR in the FTD/TPI group was significantly higher than that in the REG group (56% vs 27%; OR: 3.45; 95% CI 1.10 to 11.70; p=0.034).
Impact of emergence of NL on DCR
In the FTD/TPI group, the DCR in the NL+ patients was significantly lower than that in the NL− in the rapid group patients (7% vs 30%; OR: 0.17; 95% CI 0.009 to 0.99; p=0.048) or that in the slow group patients (7% vs 47%; OR: 0.08; 95% CI 0.004 to 0.44; p=0.0017). In the REG group, the DCR in the NL+ patients was lower than that in the NL− patients in the rapid group (14% vs 33%; OR: 0.33; 95% CI 0.05 to 1.39; p=0.14) or that in the patients in the slow group (14% vs 26%; OR: 0.48; 95% CI 0.069 to 2.03; p=0.34).
DCR at various cut-off values of TGR
In the slow group, the FTD/TPI group had a higher DCR than that of the REG group regardless of the TGR cut-off value, whereas in the rapid group, the DCR of the REG group was similar to or better than that of the FTD/TPI group for TGR cut-off values of >0.3%/day. The DCR in the REG group was maintained near 30% both in the rapid and slow groups regardless of the TGR cut-off value (online supplementary figure 2).
esmoopen-2019-000584supp004.pdf (75.9KB, pdf)
Discussion
In this retrospective study, in patients with low TGR and NL− in preceding treatment, the DCR in the FTD/TPI group was higher than that in the REG group, which suggested that patients with slow progression are better candidates for FTD/TPI than those for REG for later-line treatment. However, considering the similar DCRs in the REG and FTD/TPI groups in patients with high TGR, both REG and FTD/TPI are equally recommended for patients with rapid progression in their preceding treatment. To the best of our knowledge, the present study is the first to compare REG and FTD/TPI according to classification by TGR and NL+ in preceding treatment.
REG and FTD/TPI have different mechanisms of action. REG is a multikinase inhibitor that targets the activity of several protein kinases.1 Therefore, the efficacy of REG may depend on signalling pathway activity but independent of tumour growth speed. Conversely, because FTD/TPI is a thymidine-based nucleic acid analogue, FTD/TPI targets unspecified DNA of cancer cells.2 FTD/TPI as later line of treatment does not result in shrinkage of the tumour; therefore, it may be difficult for this treatment to control rapid-growing tumours. In addition, the time from the initiation of first-line chemotherapy to the initiation of REG or FTD/TPI may affect the TGR. In the present study, 88 (49%) of 178 patients for whom this time was ≥18 months were classified into the slow group compared with 23 (35%) of the 66 patients for whom this time was <18 months (p=0.04). In the CORRECT and CONCUR studies, which compared REG with placebo, the HR of the subgroup of patients for which the time from the initiation of first-line chemotherapy to the initiation of REG was ≥18 months was similar to that of the subgroup for which the time was <18 months (CORRECT study: 0.82 vs 0.76; CONCUR study: 0.31 vs 0.33).2 7 Conversely, in the RECOURSE and TERRA studies, which compared FTD/TPI with placebo, the HR of the subgroup of patients for which the time from the initiation of first-line chemotherapy to the initiation of FTD/TPI was ≥18 months was lower than that of the subgroup for which the time was <18 months (RECOURSE study: 0.64 vs 0.84; TERRA study, 0.67 vs 0.87).2 8 These results were consistent with those for efficacy in the present study.
There were several limitations in the present study. First, this study was a retrospective non-randomised analysis, so there was the potential for uncontrolled biases in the multivariate analyses. In addition, the number of patients included was relatively low. Therefore, rigid conclusions cannot be drawn from the results of our study. Second, it was unclear whether the method for calculating TGR and the TGR cut-off value in the study were appropriate. However, we confirmed that the concordance rate between the present and previous studies9 for the classification into the rapid and slow groups was 98% (κ=0.96), and our results remained unchanged regardless of the TGR cut-off value. In addition, our method for calculating TGR, based on the volume difference, was simpler than that of the previous report.9 Third, the intervals between the two CT scans used to assess TGR and the intervals from the initiation of REG or FTD/TPI to the first CT evaluation were heterogeneous because these were determined by the attending physicians according to local clinical practice. However, we confirmed that differences in the intervals between the scans did not affect the allocation of patients into the rapid and slow groups, and the intervals between the initiation of treatment and the first CT scan were similar between the patients receiving REG and FTD/TPI in the rapid and slow groups. However, given these limitations, further validation studies are needed to confirm the value of TGR for drug selection that may contribute to providing valuable clinical information.
In conclusion, TGR and NL during preceding treatment may be helpful for drug selection in patients with refractory mCRC to be treated with REG or FTD/TPI. However, further studies are needed to confirm the value of TGR in drug selection.
Footnotes
Contributors: The conception of the work: all authors. The design of the work: ToM, HT, TK and YK. The acquisition of data: ToM, TK and YK. The analysis of data: KO. The interpretation of data: KO, ToM and HT. Drafting the work or revising it critically for important intellectual content: all authors. Approval of the version published: all authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: The authors declare the following conflicts: ToM has received honoraria from Takeda, Chugai, Merck Serono, Taiho, Bayer, Lilly Japan, Yakult Honsha and Sanofi and has received research funding from Yakult Honsha, MSD, Daiichi Sankyo and Ono; Hiroya Taniguchi has received honoraria from Takeda, Chugai, Merck Serono, Taiho, Bayer, Lilly Japan and Yakult Honsha, Sanofi; TK has received honoraria from Taiho; YK has received honoraria from Taiho, Daiichi Sankyo, Takeda, Chugai, Merck Biopharma and Eli Lilly Japan; SK has received honoraria from Chugai, Bristol-Myers Squibb, Ono, Merck KGaA, Bayer, Lilly and Yakult Honsha, and research funding from Taiho, Bristol-Myers Squibb, Ono, Lilly and MSD; MT has received honoraria from EA pharma and Olympus and has received research funding from EA pharma; HN has received honoraria from Takeda, Chugai, Ono, Bayer and Lilly Japan; SY has received honoraria from Takeda, Chugai, Bristol-Myers Squibb, Ono, Merck Serono, Taiho, Bayer, Lilly Japan, Yakult Honsha and Sanofi; KM has received honoraria from Takeda, Chugai, Taiho, Bayer, Eli Lilly, Ono, Sanofi and Bristol-Myers Squibb and has received research funding from Parexel International, Merck Serono, MSD, Mediscience Planning, Pfizer, Daiichi Sankyo, Sanofi, Shionog, Sumitomo Dainippon Pharma, Solasia Pharma and Takeda; YK has received honoraria Takeda, Chugai, Bristol-Myers Squibb, Ono, Merck Biopharma, Taiho, Bayer, Lilly, Yakult Honsha, Sanofi, Nipro, Moroo, Asahi Kasei, Mitsubishi Tanabe, Otsuka, Medical Review and Shiseido and has received research funding from MSD, Daiichi Sankyo, NanoCarrier, Eisai, Sysmex, Shionogi, IQVIA, Parexel International, Astellas, Mediscience, Sumitomo Dainippon, A2 Healthcare, Ono, Taiho, Bayer, Yakult Honsha and Sanofi; KY has received honoraria from Takeda, Chugai, Daiichi Sankyo, Bristol-Myers Squibb, Ono, Merck Serono, Taiho, Bayer, Lilly, Yakult Honsha, Sanofi and MSD and has received research funding from Taiho.
Patient consent for publication: Not required.
Ethics approval: The Institutional Review Boards of the Aichi Cancer Center Hospital (approval number: 2017-1-034), the Institutional Review Boards of Shizuoka Cancer Center (approval number: T26-59-28-1-3) and the Institutional Review Boards of Hokkaido University Hospital (approval number: 017-0017).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1.Grothey A, Van Cutsem E, Sobrero A, et al. . Regorafenib monotherapy for previously treated metastatic colorectal cancer (correct): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 2.Mayer RJ, Van Cutsem E, Falcone A, et al. . Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 3.Masuishi T, Taniguchi H, Hamauchi S, et al. . Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: a retrospective comparison. Clin Colorectal Cancer 2017;16:e15–22. 10.1016/j.clcc.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 4.Moriwaki T, Fukuoka S, Taniguchi H, et al. . Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese Society for cancer of the colon and rectum multicenter observational study. Oncologist 2018;23:7–15. 10.1634/theoncologist.2017-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atallah S, Cho BCJ, Allibhai Z, et al. . Impact of pretreatment tumor growth rate on outcome of early-stage lung cancer treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;89:532–8. 10.1016/j.ijrobp.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 6.van Bockel LW, Verduijn GM, Monninkhof EM, et al. . The importance of actual tumor growth rate on disease free survival and overall survival in laryngeal squamous cell carcinoma. Radiother Oncol 2014;112:119–24. 10.1016/j.radonc.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 7.Li J, Qin S, Xu R, et al. . Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (concur): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. 10.1016/S1470-2045(15)70156-7 [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Kim TW, Shen L, et al. . Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol 2018;36:350–8. 10.1200/JCO.2017.74.3245 [DOI] [PubMed] [Google Scholar]
- 9.Ferté C, Fernandez M, Hollebecque A, et al. . Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res 2014;20:246–52. 10.1158/1078-0432.CCR-13-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000584supp001.pdf (54.9KB, pdf)
esmoopen-2019-000584supp002.pdf (22KB, pdf)
esmoopen-2019-000584supp003.pdf (22.1KB, pdf)
esmoopen-2019-000584supp004.pdf (75.9KB, pdf)