-
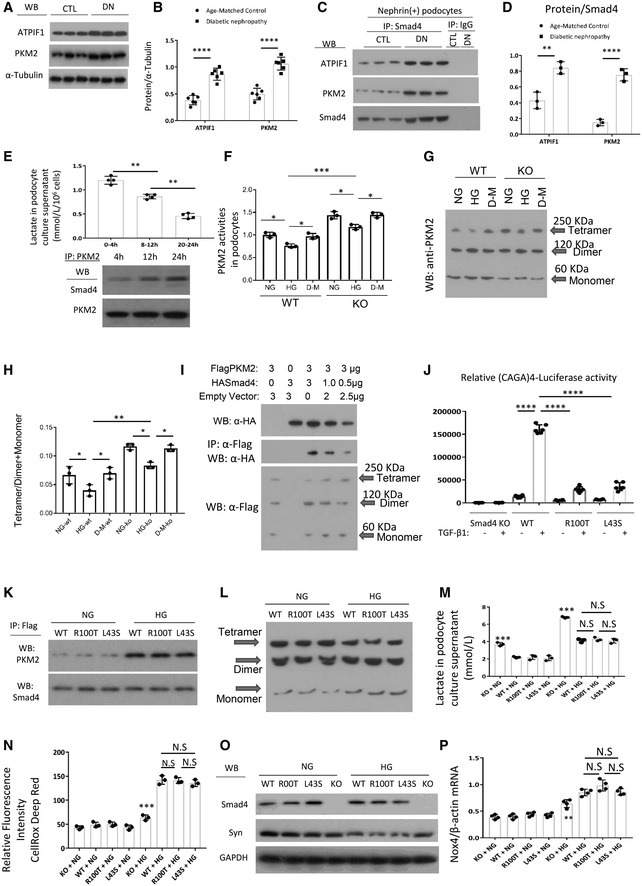
A
WB demonstrated expression levels of ATPIF1 and PKM2 in age‐matched kidneys and 16‐week type 2 diabetic nephropathy.
-
B
Quantitation of arbitrary ratios of ATPIF1 and PKM2 to α‐tubulin.
-
C
Immunoprecipitation (IP)/WB demonstrated the interactions between Smad4 and ATPIF1 and PKM2 in nephrin+ podocytes isolated from age‐matched kidneys and 16‐week type 2 diabetic nephropathy.
-
D
Quantitation of arbitrary ratios of ATPIF1 and PKM2 to Smad4.
-
E
Lactate production in different periods under high glucose treatment in podocytes (upper panel), IP/WB demonstrated interaction between Smad4 and PKM2 after 4‐, 12‐ and 24‐h HG treatment in podocytes (lower panel).
-
F
PKM2 activities in wild‐type (WT), Smad4 KO (KO) mouse podocytes under normal glucose (NG), high glucose (HG) or D‐manitol (D‐M) treatment for 24 h.
-
G
Western blotting demonstrated PKM2 tetramer, dimer and monomer after normal glucose (NG), high glucose (HG) or D‐manitol (D‐M) treatment for 24 h in wild‐type (WT) or Smad4 KO (KO) mouse podocytes after cross‐linking treatment.
-
H
Quantification of ratios of Tetramer/Dimer+Monomer.
-
I
293T cells were transduced with FlagPKM2, HASmad4 and empty vector with various dosages. After 48 h, cells were collected for Western blotting (upper panel), immunoprecipitation/Western blotting (middle panel) and cross‐linking/Western blotting (lower panel). Western blotting demonstrated expression levels of HA‐Smad4 (upper panel), interactions of FlagPKM2 with HASmad4 (middle panel) and FlagPKM2 tetramer, dimer and monomer (lower panel).
-
J
Smad4 KO podocytes were transduced with an empty retroviral vector, or retroviral vectors over‐expressing Smad4 WT, Smad4 R100T or Smad4 L43S. SBE4‐Luciferase assay demonstrated transcription activities in Smad4 KO, Smad4 WT, Smad4 R100T and Smad4 L43S podocytes with or without TGF‐β1 treatment.
-
K
IP/WB demonstrated interaction between Smad4 and PKM2 after 24‐h NG or HG treatment in WT, R100T and L43S podocytes.
-
L
Cross‐linking/Western blotting demonstrated PKM2 tetramer, dimer and monomer after 24‐h NG or HG treatment in WT, R100T and L43S podocytes.
-
M
Lactate production in WT, R100T, L43S and Smad4 KO podocytes after 24‐h NG or HG treatment.
-
N
CellRox Deep Red test demonstrated relative fluorescence intensity in WT, R100T, L43S and Smad4 KO podocytes treated with NG or HG for 24 h.
-
O
WB demonstrated expression levels of synaptopodin, Smad4 and GAPDH in WT, R100T, L43S and Smad4 KO podocytes treated with NG or HG for 24 h.
-
P
RT–qPCR demonstrated ratios of Nox4/β‐actin in WT, R100T, L43S and Smad4 KO podocytes treated with NG or HG for 24 h.
Data information: One‐way ANOVA; all values are shown as means ± SD of at least three independent experiments. *
< 0.0001. In (M, N and P) **
< 0.001 versus WT or versus R100T or L43S under NG or HG condition. N.S, not significant,
> 0.05.