Summary
Primary biliary cholangitis (PBC) is an autoimmune, cholestatic, chronic liver disease that ultimately progresses towards cirrhosis and liver failure if untreated. While ursodeoxycholic acid has been established as standard of care for PBC in the last few decades, significant advances in second-line treatment options have recently been made and new therapeutic developments are currently under evaluation. The purpose of this article is to provide the clinician with an overview of the current treatment options and future opportunities for patients with PBC.
Keywords: PBC, ursodeoxycholic acid, obeticholic acid, fibrates, FXR, PPAR, cholestasis, inflammation, liver disease
Key points
Greater understanding of the pathophysiology of PBC will lead to new therapeutic opportunities
The current standard of care for PBC is treatment with ursodeoxycholic acid
Some patients with PBC respond poorly to ursodeoxycholic acid and therefore require second-line therapies
A number of promising second-line therapies are currently in development for patients with PBC
Alt-text: Unlabelled Box
Introduction
Primary biliary cholangitis (formerly termed primary biliary cirrhosis, PBC) is a rare disease (occurring in less than 1/2,000) mainly diagnosed in women. It is characterised by an autoimmune and inflammatory process targeting the interlobular bile ducts. Without treatment the disease generally progresses to cirrhosis and liver failure over a period of 10 to 20 years. In the eighties, liver transplantation was the only therapeutic resource for patients with PBC. A major landmark in the history of PBC was the introduction of ursodeoxycholic acid (UDCA) as an effective therapy. After many years of controversy, there is now a consensus to accept UDCA as the standard therapy. UDCA has been shown to extend transplantation–free survival, especially when started early in the course of the disease.[1], [2], [3], [4]
The severity of PBC varies widely, young patients (less than 40-years old at onset) tend to progress more rapidly towards cirrhosis. The notion of an optimal biochemical response to UDCA therapy has been an important step towards assessing the severity of the disease and stratifying patients according to their risk of progressing towards liver failure.[5], [6], [7], [8], [9] About 30% to 40% of patients will not have an optimal biochemical response to UDCA. These patients exhibit more rapid disease progression than those with normalisation of serum alkaline phosphatase (ALP), aminotransferases and bilirubin. In a meta-analysis of 4,845 patients with PBC, the strongest predictors of death or liver transplantation were ALP levels more than 1.5–3x the upper limit of normal range and abnormal serum bilirubin.10 These markers are now considered the gold standard of surrogate biochemical endpoints for clinical trials of novel therapies.
From pathophysiology to therapeutic opportunities
Despite relentless efforts to understand the autoimmune pathogenesis of PBC, to date, no potentially effective new immunotherapies have emerged. PBC is characterised by multilineage immune dysregulation, which is related to the loss of tolerance to the E2 component of the mitochondrial oxo-dehydrogenase pathway (mainly PDC E2). As in other autoimmune diseases, loss of peripheral tolerance might result from several non-mutually exclusive mechanisms involving predisposing genes, epigenetic changes, gender, chemicals, infections, molecular mimicry, tissue damage and inflammation.
Cholangiocytes as culprit and victim in the initiation and progression of PBC
A milestone in the pathophysiology of PBC was the discovery of defective AE2 [SLC4A2] expression and biliary HCO3− secretion in patients with PBC, and its reversal by UDCA therapy.[11], [12] According to the bicarbonate « umbrella » theory, defective AE2 expression and reduced biliary bicarbonates might be responsible for the enhanced susceptibility of cholangiocytes to damage from biliary bile acids.13 In addition to the lack of a bicarbonate protective barrier, the reduced alkalinity of bile in PBC might impair the physiological protective function of biliary ALP against danger/pathogen-associated molecular pattern-induced biliary inflammation and stress.14
Recently, it has been shown that microRNA-506 (miR-506) on chromosome X is an important player in the cellular mechanisms leading to the defective bicarbonate secretion, cholestasis and immune dysregulation observed in PBC. MiR-506 is overexpressed in PBC cholangiocytes and directly targets both AE2 and type III inositol 1,4,5-triphosphate receptor. MiR-506 induces downregulation of biliary and epithelial markers together with upregulation of mesenchymal, proinflammatory and profibrotic markers, as well as increasing oxidative and endoplasmic stress, and sensitising cholangiocytes to apoptosis.[15], [16]
Further evidence suggests that senescence, dysregulated autophagy and apoptosis are among cholangiocytes’ responses to oxidative stress and injury in PBC.17 All these modes of response are associated with abnormal expression and presentation of mitochondrial antigens to immune cells. Indeed, these cholangiocyte responses might act as a downstream amplifying mechanism in the autoimmune process.18
In brief, the combination of genetic susceptibility, abnormal adaptive responses to cholangiocyte injury, and epigenetic alterations may contribute to disease initiation and progression. According to current knowledge, protection of cholangiocytes by drugs (mainly UDCA, 24-norUCDA,19 Farnesoid X receptor [FXR]/Takeda G protein-coupled receptor 5 [TGR5] agonists) that restore alkaline choleresis is essential. Targeting miR-506 deserves major attention as a novel therapeutic opportunity.
Cholestasis and hepatocellular injury: the second hit in the disease progression
Hepatocellular retention of endogenous bile acids (cholestasis) is the second hit thought to induce subsequent hepatocyte inflammation and destruction, and to promote fibrosis progression leading to end-stage disease. Compounds that reduce liver bile acid overload may be effective, as shown by the therapeutic effects of peroxisome proliferator-activated receptor (PPAR) and FXR (or NR1H4) agonists, as well as preliminary data with fibroblast growth factor 19 (FGF19) enterokine analogues. Whether blockers of intestinal apical bile salt transporter and the entry of bile acids into hepatocytes (myrcludex B) might be useful remains to be explored.20
Finally, liver fibrosis in PBC results from both cholangiocellular and hepatocellular injury.21 In this setting, the fibrogenic process appears to be related to complex interactions between immune/inflammatory mechanisms, cytokine networks and the derangement of homeostasis between epithelial and mesenchymal cells, which could explain the current lack of efficacious antifibrotic drugs.[22], [23]
Current landscape of PBC therapies
First-line treatment
Despite the emergence of competing drugs, UDCA is still recognised as the universal first-line standard of care for PBC.[1], [2] This strong recommendation is based on the long-term efficacy of UDCA and its excellent safety profile, together with its low cost. Historically, UDCA was the first drug to show efficacy in treating PBC in a placebo-controlled trial.[24], [25] At a daily dose of 13–15 mg/kg, it has been associated with a consistent improvement of biochemical features of cholestasis, in particular of serum levels of ALP and of total bilirubin, 2 major prognostic markers in PBC.[26], [27], [28], [29], [30] Some immunological indices of the disease, such as serum level of immunoglobulin M (IgM), and antimitochondrial antibody titre have also been shown to decrease in response to UDCA. In addition, there is evidence to suggest that UDCA is able to slow down histological progression and the development of portal hypertension.[31], [32], [33] However, because of the slow progression of the disease, it is noteworthy that none of the phase III trials of UDCA in PBC was individually powered to evaluate hard clinical outcomes such as death or liver transplantation (LT). Open-label extension studies were able to show that 4-year UDCA therapy was associated with a lower probability of death or LT compared to a 2-year delay in UDCA introduction.[25], [34] After contradictory meta-analyses and extensive debates, UDCA has been recognised to slow disease progression and to reduce mortality and the need for LT, this effect being more significant when UDCA is started at early stages of disease and is continued for life.[4], [35] In clinical practice, UDCA therapy is associated with an excellent tolerability profile. Definitive discontinuation due to digestive adverse effects (diarrhoea, abdominal discomfort) is reported in less than 5% of patients.36
Second-line treatment options
Obeticholic acid
After UDCA, obeticholic acid (OCA) was the second drug to successfully meet the primary endpoint of a large placebo-controlled phase III trial in PBC. This goal was achieved both in patients with incomplete response or intolerance to UDCA and in UDCA-naive patients.[37], [38] OCA is a synthetic bile acid derivative with a high affinity for FXR, a nuclear receptor that closely regulates bile acid synthesis and secretion, and has been shown to mediate anti-inflammatory and antifibrotic effects.[39], [40] OCA is the first FXR-selective agonist approved as a therapeutic product in humans. OCA was conditionally approved by the US and EU regulatory authorities in 2016 for the treatment of PBC in patients with incomplete response or intolerance to UDCA. This approval, based on the results of the POISE study, a 1-year placebo-controlled trial with an extended 1-year open-label extension (OLE) period, is conditional on further evaluation in an ongoing phase IV trial (COBALT) to determine if the biochemical benefits associated with OCA therapy will have a direct impact on clinical outcomes/quality of life in patients with PBC.37
The characteristics of the POISE study are summarised in Table 1. Inclusion criteria included a suboptimal biochemical response (i.e. ALP level ≥1.67x ULN or abnormal level of total bilirubin, but ≪2 mg/dl, after 1 year of UDCA) or intolerance to UDCA. A total of 216 patients were randomly assigned to OCA at a 10 mg daily dose (10 mg group), OCA at a dose of 5 mg with adjustment to 10 mg if applicable after 6 months (5–10 mg group), or placebo. In total, 93% of patients received UDCA (13–15 mg/kg/d) as background therapy. The primary outcome was an ALP level ≪1.67x ULN with a drop of ≥15% from baseline, and a normal bilirubin level. It occurred in 46% of patients in the 5–10 mg group, 47% in the 10 mg group, and 10% in the placebo group (p ≪0.001 for both comparisons). Compared to placebo, OCA was associated with a significant reduction in serum levels of ALP and total bilirubin (approximately -35% and -8%, respectively, from baseline to month 12). The levels of gamma-glutamyltransferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), conjugated bilirubin, and total bile acid also decreased significantly in each OCA group compared to placebo. Changes in non-invasive measures of liver fibrosis, including liver stiffness assessed by transient elastography and enhanced liver fibrosis (ELF™) score, did not differ significantly between either OCA group or the placebo group at 12 months. Pruritus was the most common adverse event reported during the double-blind phase of the study. It was 1.5–2-fold more common with OCA than with placebo, occurring in up to 68% of patients treated with OCA compared to 38% of patients receiving placebo. In the 5–10 mg arm, pruritus worsened in patients with pre-existing pruritus, but not in the patients without pruritus at baseline. The incidence and intensity of pruritus, and the rate of treatment discontinuation due to pruritus were consistently higher in the 10 mg group than in the 5–10 mg group. However, mainly as a result of OCA dose reductions, changes in pruritus scores tended to decrease over time with no significant difference observed at 12 months between either OCA group and the placebo group. The rate of serious adverse events was 16% in the 5–10 mg group, 11% in the 10 mg group, and 4% in the placebo group.
Table 1.
Characteristics of the 2 recent positive pivotal trials of second-line therapy for PBC.
| POISE Study | BEZURSO Study | |
|---|---|---|
| Study design | ||
| Drug | Obeticholic acid | Bezafibrate |
| Arms | 5-10 mg/10 mg/placebo | 400 mg/placebo |
| Randomization | 1:1:1 | 1:1 |
| Entry criterion | Toronto (rev.) non-response | Paris-2 non-response |
| Double-blind period | 12 months | 24 months |
| Primary outcome | ALP decrease ≥15% from baseline, ALP ≪1.67x ULN and normal bilirubin | Normal levels of total bilirubin, ALP, AST, ALT, albumin, and PI |
| Study population at baseline | ||
| Patients enrolled | 217 | 100 |
| Age (year) | 56 | 53 |
| Pruritus | 59% | 66% |
| Total bilirubin (mg/dl) | 0.65 | 0.78 |
| ALP Level (U/L) | 323 | 277 |
| LSM (kPa) | 11.6 | 12.1 |
| Cirrhosis | 19% | 21% |
| UDCA-untreated patients | Yes (7%) | No |
| Study outcomes | ||
| Completed study | 91% | 92% |
| Primary outcome (per arm) | 46%/47%/10% | 31%/0% |
| Normal ALP level (per arm) | N/A | 67%/2% |
| OR of Paris-2 response | 9.1/8.5 | 21.7 |
| Reduction in pruritus | No | Yes |
| Reduction in LSM | No | Yes |
| Reduction in ELF score | No | Yes |
| Significant side effects | Pruritus | 5% creatinine increase |
Data are expressed as number, % or mean. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELF™, Enhanced Liver Fibrosis; LSM, liver stiffness measurement; OR, odds ratio; PI, prothrombin index (a derived measure of prothrombin time); ULN, upper limit of normal. Toronto (rev.) non-response: ALP ≫1.67x ULN or abnormal total bilirubin. Paris-2 non-response: ALP ≫1.5x ULN or AST ≫1.5x ULN or abnormal total bilirubin.
The results and safety profile observed during the 12-month OLE period were consistent with those reported during the double-blind phase of the trial. Durable responses in the main serum markers of cholestasis were reported through 48 months of the OLE study in both patients treated with OCA from the double-blind phase and those initially treated with placebo then switched to OCA.41 In this long-term OLE study, pruritus was the most common side effect of OCA (reported in 77% of patients), but discontinuation due to pruritus was reported in only 4% of patients. In a POISE sub-study, the effect of 3-year OCA therapy on liver fibrosis progression was assessed in 13 patients with paired liver biopsies.42 In this small population, histological fibrosis stage only worsened in 2 (15%) patients, suggesting that OCA may slow fibrosis progression in patients with high-risk PBC.
Fibrates
Bezafibrate is the third treatment, after UDCA and OCA, to have shown clear beneficial effects in a large, well-powered, placebo-controlled trial in PBC.43 Fibrates have been recognised since the 1960s as lipid lowering agents. They are potent agonists of the PPAR-α, a transcription factor involved in fatty acid catabolism and inflammatory response. Some fibrates, such as bezafibrate, further exhibit additional affinities for PPAR-δ and PPAR-γ, the other 2 isoforms of PPARs involved in energy metabolism and inflammatory processes.44 Fibrates are also known to repress bile acid synthesis in the liver and to increase phospholipid excretion into the bile.[45], [46]
In 1999, Iwasaki et al. were the first to report an improvement of the biological parameters of PBC, including ALP, GGT and IgM serum levels, with bezafibrate.47 This observation was subsequently confirmed by numerous small, unblinded studies, first conducted in Japan, then replicated in Western countries, either with bezafibrate or with fenofibrate.[48], [49], [50], [51], [52], [53] The BEZURSO study (bezafibrate in combination with ursodeoxycholic acid in primary biliary cholangitis, NCT01654731) is the first and currently the only available placebo-controlled phase III trial aimed to assess the efficacy and safety of a fibrate in PBC.43 The characteristics of this trial are summarised in Table 1, alongside those of the POISE study.
The BEZURSO study randomly assigned 100 patients who had an inadequate biochemical response to UDCA based on the Paris-2 criteria (i.e. ALP or AST levels ≫1.5x ULN, or elevated total bilirubin) to receive bezafibrate at a daily dose of 400 mg (50 patients), or placebo (50 patients), in addition to continued treatment with UDCA (13–15 mg/kg/d) for 24 months. The primary endpoint, defined as normal levels of bilirubin, ALP, aminotransferases, albumin, and prothrombin index (a derived measure of prothrombin time) at 24 months, was achieved in 31% of patients in the bezafibrate group and 0% in the placebo group (p ≪0.001). At 24 months, normal levels of ALP were observed in 67% of patients in the bezafibrate group and in 2% in the placebo group (p ≪0.001). Changes in total bilirubin, ALP, GGT, and aminotransferases were consistent with the result of the primary endpoint, as were the parallel changes in pruritus, fatigue, and non-invasive markers of liver fibrosis, including liver stiffness measurement and ELF score. Reduction in pruritus intensity, which was reported in previous studies,[45], [51] did not correlate with serum measures of autotaxin, a lysophospholipase previously shown to play a role in the pathogenesis of cholestatic pruritus.54 Serum IgM levels decreased by 21% in the bezafibrate group and only 2% in the placebo group, but the difference did not reach the level of significance. The C4 bile acid precursor, a serum marker of bile acid synthesis, significantly decreased with bezafibrate, in parallel with the circulating fraction of endogenous bile acids. Like the POISE study, the BEZURSO study was not sufficiently powered to assess long-term clinical outcomes. The incidence of liver-related complications at 24 months did not differ between groups (2 clinical events in each group, no death).
The overall incidence rate of serious or non-serious adverse events did not differ between groups. Myalgia was more frequently observed in the bezafibrate group than in the placebo group (20% vs. 10%, respectively) but the difference was not significant. Bezafibrate was associated with a significant increase in serum creatinine level of 5% at 24 months, a well-known class effect of fibrates with no long-term impact on renal function.55 Patients with features of portal hypertension or high ALP levels at baseline were less likely to respond to bezafibrate and to meet the primary endpoint. The application of the GLOBE and UK-PBC risk scores showed significantly lower projected mortality or need for LT at 5, 10, and 15 years in the bezafibrate group than in the placebo group.56 Mortality or need for LT was predicted to increase by 15%–25% with placebo and to decrease by 30%–40% with bezafibrate. At the end of study, the differences in estimated 15-year outcomes between bezafibrate and placebo groups were −57% (95% CI −77% to −36%) with the UK-PBC score and −46% (95% CI −65% to −27%) with the GLOBE score.
Budesonide
Historically, budesonide was the first second-line treatment in PBC to show promising results in association with UDCA.[57], [58] Recent data, however, have been somewhat disappointing.59 Budesonide is a non-halogenated corticosteroid with potent dual agonism for the glucocorticoid and xenobiotic pregnane X receptors (PXR), which exhibits a high (90%) first-pass effect through the liver when administered orally, thus limiting systemic bioavailability and related side effects.60 The latter property is of specific relevance when considering the high risk of osteoporosis in women with PBC. Importantly, budesonide is inadvisable in cirrhotic patients in whom its first-pass effect is impaired, exposing them to significantly higher plasma concentrations and increased risk of systemic side effects. Oral budesonide is presently a licensed indication for patients with Crohn’s disease, ulcerative colitis and autoimmune hepatitis.
The first 2 randomised controlled trials of budesonide in PBC, one of which was unblinded, led to the same observation that at least 2 years of budesonide (6 mg/d) in association with UDCA (13–15 mg/kg/d) was associated with a significant improvement in histological activity and fibrosis scores, thus suggesting that budesonide can slow PBC progression.[57], [58] Budesonide was also shown to improve serum levels of ALP, aminotransferases, and IgM significantly, indicating that it was able to improve both cholestatic and immunological features of the disease.[57], [61] However, it should be noted that neither of these first 2 controlled trials was based on targeted at-risk populations. In a recent double-blind, placebo-controlled, still unpublished trial, 62 non-cirrhotic patients with PBC and an incomplete biochemical response to UDCA (defined by an ALP level ≫1.5x ULN) and a liver biopsy compatible with PBC and showing inflammatory activity within 6 months prior to baseline, were randomly assigned 2:1 to oral budesonide 9 mg/d (n = 40) or placebo (n = 22), with continued UDCA (12–16 mg/kg/d), for 36 months.59 Adjusting the dose of budesonide down to 3 mg/d was allowed in patients with normal AST levels. Not all patients had a control liver biopsy (n = 43) or completed 3 years of treatment (n = 29). The primary endpoint, an improvement of ≥3 points in Ishak inflammatory activity score or no inflammation and no progression of fibrosis stage, did not differ between groups (43% in the budesonide group vs. 29% in the placebo group; p = 0.225), but the proportion of patients with serum ALP levels ≪1.67x ULN and a ≥15% drop from baseline and normal bilirubin was significantly higher in the budesonide group than in the placebo group at 12, 24, and 36 months (40% vs. 18%, 45% vs. 18%, and 43% vs. 23%, respectively; p ≪0.05 for each comparison). Adverse events were reported in a similar number of patients across groups. Adverse drug reactions were reported for 24 patients (60%) in the budesonide- and 8 patients (36%) in the placebo group. Budesonide was associated with significantly reduced serum cortisol concentration and lumbar spine bone density.
In summary, while this latter study was negative with respect to the primary histological endpoint, clinically meaningful improvements in biochemical markers of disease activity were apparent and tolerability was consistent with prior clinical experience. Since studies using liver histology in PBC are challenging to achieve, a lack of power may explain these contrasting results. Accordingly, budesonide should still be considered as a potentially interesting candidate for second-line therapies in PBC.
Treatment algorithm
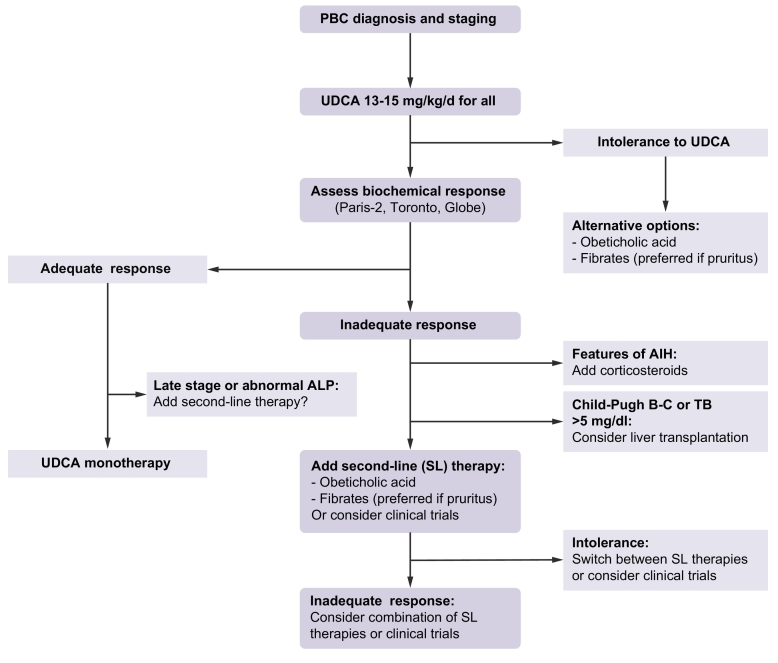
An inadequate biochemical response to UDCA is one of the strongest determinants of clinical outcomes in PBC.[5], [10] Therefore, it has been recommended that second-line therapy be considered specifically in the patients who experience such an incomplete response (Fig. 1).[1], [2] The criteria used to define suboptimal response to UDCA (Paris-2, Toronto, GLOBE, Barcelona, etc.) can differ among teams and practitioners provided that they include both ALP and bilirubin levels. The European Association for the Study of the Liver recommends considering an ALP level ≫1.5x ULN or abnormal levels of total (and conjugated) bilirubin as biochemical thresholds above which second-line treatment or inclusion in clinical trials should be considered.1 It is expected that more stringent criteria will be used in the future, as complete normalisation of ALP has been shown to provide additive value in predicting outcomes.62 Advanced disease stage or progressive increase in liver stiffness, 2 other major predictors of clinical outcomes in PBC, are not currently taken into account in decision-making, but probably should be.63 To date, OCA and bezafibrate (fenofibrate by extension) are the 2 drugs that should be considered first for second-line therapy in PBC, as both of them have proven successful in properly powered phase III trials and selected high-risk patients.[37], [43] Available data on fenofibrate suggest that beneficial effects comparable to those of bezafibrate can be expected with this drug, making it particularly attractive in countries in which bezafibrate is not available like in the US.[64], [65] While the use of fibrates in Western countries is still off-label for PBC, OCA benefits from a conditional approval by US and EU authorities. However, without regard to any regulatory and price considerations and in the absence of comparative studies, the choice between OCA and fibrates must rely on several parameters, among which are the local availability of the drug, the presence and intensity of pre-treatment pruritus, the presence and intensity of pre-treatment myalgia, the presence of pre-treatment renal dysfunction or dyslipidaemia, and the physician’s own experience and confidence.
Fig. 1.
Treatment algorithm for PBC.
Initial staging of PBC should preferably be based on non-invasive measures including total bilirubin, ALP, aminotransferases, albumin, platelet count, liver stiffness measurement, and liver ultrasound. All patients must be treated with UDCA as a first-line treatment. Intolerance to UDCA (diarrhoea, stomach burns) may occur rarely (5%). Alternative options (OCA or fibrates) should then be considered. Assessment of biochemical response to UDCA is typically performed at 12 months of UDCA, but earlier evaluation after as few as 6 months of UDCA therapy may be proposed in patients with the most severe or symptomatic (pruritus) disease. The response criteria used must include ALP and bilirubin levels (Paris-2, Toronto, GLOBE, etc.). Abnormal levels of total and conjugated bilirubin or ALP level ≫1.5x ULN are minimal thresholds above which second-line therapies should be considered. Patients with adequate biochemical response to UDCA can be kept on UDCA monotherapy. Advanced-stage responders or those with persisting abnormal ALP might be considered for second-line therapies. In poor biochemical responders, liver biopsy should be considered when AIH-PBC variant or any other hepatic comorbidity is suspected. Addition of corticosteroids (including budesonide) is recommended in patients with AIH-PBC variant. Patients with non-regressive jaundice (bilirubin ≫5 mg/dl) or features of advanced cirrhosis (Child-Pugh B-C) should be referred for liver transplant. All remaining poor responders to UDCA should be considered for second-line therapies (i.e. OCA or fibrates) in addition to continued UDCA. Fibrates should be preferred in patients with pruritus. Second-line therapies could be switched in case of poor tolerance (pruritus for OCA; myalgia for fibrates) or combined in case of insufficient response.
AIH, autoimmune hepatitis; ALP, alkaline phosphatase; OCA, obeticholic acid; PBC, primary biliary cholangitis; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Therapeutic perspectives in the near future
A number of other options are currently under investigation for the treatment of non-responders to UDCA, with or without OCA or fibrates. Generally speaking and as already discussed, therapeutic opportunities in PBC are offered by targeting the so-called “upstream” immune response, “midstream” biliary injury leading to cholestasis and “downstream” fibrotic processes.66 Novel therapeutic approaches target mainly cholestasis (which has a central role in disease progression) but a number of these primarily anti-cholestatic agents have multiple potential targets including anti-inflammatory, antifibrotic, immunomodulatory and metabolic effects. No single agent will be universally effective, so individualisation of therapy and smart combinations are likely to be required in the future. Stratification and timing of therapy are crucial. Nevertheless, we are approaching promising times for patients with PBC, provided that the off-target effects of these novel drugs are mild. The following paragraphs provide an overview of the most advanced therapeutic candidates under evaluation (the main available results are summarised in Table 2).
Table 2.
Main published studies (or presented with a minimum 52 week-follow-up) of drugs in development for PBC.
| Main Target | Agent | Study design | Inclusion criteria | Duration | No. patients | Main results |
|---|---|---|---|---|---|---|
| Immunity | Ustekinumab67 | Open-label | Inadequate response to UDCA | 28 wk | 20 | Modest decrease in ALP (-12%) |
| Immunity | Rituximab (1,000 mg x2)65 |
Open-label | Inadequate response to UDCA | 6-10 mo | 14 | Modest decrease in ALP (-16%) at 6 months |
| Immunity | Rituximab (1,000 mg x2)66 | Randomised Placebo-controlled phase II | Moderate or severe fatigue | 12 mo | 57 | No improvement in fatigue score |
| Cholestasis | Seladelpar (50 or 200 mg/d)68 |
Randomised Placebo-controlled phase II | Inadequate response to UDCA | 12 wk | 70 | -53% to -63% decrease in ALP 3 grade-3 ALT increases |
| Cholestasis | Seladelpar (5-10 or 10 mg/d)69 |
Randomised dose ranging phase II | Inadequate response to UDCA | 52 wk | 119 (results in 34) |
-47% and -46% decrease in ALP No ALT flare |
| Cholestasis | NGM28272 | Randomised Placebo-controlled phase II | Inadequate response to UDCA | 4 wk | 45 | -16% to 19% decrease in ALP Diarrhoea in 1/4 |
| Cholestasis | GSK233067276 | Placebo-controlled, cross-over, phase II | Pruritus | 2 wk | 22 | Significant decrease in itch scores Diarrhoea in 1/3 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; PBC, primary biliary cholangitis; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Immunological agents
Targeting the upstream immune response that is thought to initiate biliary epithelial cells makes sense. Unfortunately, broadly acting immunosuppressive therapies have proven disappointing to date (at least when used in a non-stratified manner). Indeed, these agents are most likely to be effective in early disease, even before it is clear that the patient is at high risk of progression. As a result, appropriate timely design of clinical trials with immunomodulatory therapy is a challenge. Recent trials of immunologic agents have included rituximab and ustekinumab.
Rituximab was the first biologic agent tested in PBC. Rituximab is a B-cell-depleting monoclonal antibody targeting the CD20 antigen. An initial open-label study, enrolling 6 patients, demonstrated a significant reduction in ALP up to 36 months after 2 doses of 1,000 mg (separated by 2 weeks).67 However, a subsequent open-label study, including 14 patients, demonstrated only a mild improvement in liver tests (although pruritus decreased in 60%),68 and a phase II randomised controlled trial with improvement in fatigue as the primary outcome failed to demonstrate efficacy at 12 months.69 The overall conclusion is that rituximab has limited efficacy in PBC and is not recommended despite a good short-term safety profile.
Because of genetic studies suggesting the importance of the IL-12 pathway, ustekinumab, a monoclonal antibody targeting IL-12 and IL-23 has been investigated in an open-label trial of 20 patients treated for 20 weeks. Only a modest decrease in ALP was observed (-12% at week 28) and the initially planned double-blind study was not initiated.70
Other immunologic agents are under investigation and include modulation of the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) pathway (abatacept)(NCT02078882), sphingosine-1-phosphate signalling (etrasimod), or blockade of CD40/CD40L (FFP104) (NCT03155932), but final results are not available yet. Orally active Janus kinase (JAK) inhibitors are well-tolerated and have shown promise in a number of extrahepatic autoimmune diseases. They represent an interesting potential therapeutic approach. A randomised, placebo-controlled study evaluating the efficacy and safety of baricitinib (LY3009104) is planned (NTCP 03742973).
Anti-cholestatic agents
Non-fibrate PPAR agonists
Beside fibrates, other PPAR agonists are under evaluation in PBC. Seladelpar is a selective PPAR-δ agonist. Whereas PPAR-α liver expression is mainly restricted to hepatocytes, PPAR-δ is expressed not only in hepatocytes but also in cholangiocytes, Kupffer cells and hepatic stellate cells. Anti-inflammatory and antifibrotic effects of PPAR-δ activation have been demonstrated in a mouse model. A placebo-controlled study of 41 patients with UDCA non-responsive PBC was stopped because 3 patients developed significant but self-limiting increases in aminotransferase levels, up to 20x the ULN in patients treated with the highest dose (200 mg/d).71 Significant decreases in ALP were observed in patients treated with seladelpar; interestingly, the 5 patients who received the intended 12-week course normalised ALP. In a recent randomised, dose ranging phase II study (5–10 mg vs. 10 mg/d) (NCT02955602), the mean decrease in ALP was similar in both groups at 52 weeks (-47% and -46%, respectively) with normalisation in a quarter of patients; the intensity of pruritus decreased and there was no aminotransferase safety signal.72 A phase III study has been initiated.
Elafibranor is a dual PPAR-α and PPAR-δ agonist that has shown promise in non-alcoholic steatohepatitis. A recent 12-week phase II study of this drug in non-cirrhotic patients with PBC and inadequate response to UDCA has shown beneficial effects on biochemical features of cholestasis and on pruritus, which make it a promising novel treatment candidate.73
Non-OCA FXR agonists
Non-steroidal agonists, including tropifexor, EDP-305 and GS9674, are under active investigation. Results of an interim analysis of a randomised, double-blind, placebo-controlled phase II study of tropifexor (NCT02516605) showed a dose-dependent decrease in GGT (-25% to -70%) at 4 weeks.74 Severe itch did not occur and further investigation over a longer treatment duration is planned. The global expectation is that non-steroidal FXR agonists will demonstrate a similar or even higher efficacy than OCA on biochemical features of cholestasis without inducing pruritus. However, further studies are required to confirm these preliminary results.75
Fibroblast growth factor 19 analogues
In response to bile acid exposure, an enterokine, FGF19, is produced by ileal enterocytes and secreted into the portal circulation. In hepatocytes, FGF19 controls bile acid metabolism via actions on CYP7A1, the rate-limiting enzyme in the classic pathway of bile acid synthesis. NGM282 is a subcutaneously administered synthetic non-tumorigenic analogue of FGF19. A 28-day, double-blind, placebo-controlled phase II trial, performed in 45 patients with an inadequate response to UDCA, demonstrated that NGM282 led to a significant decrease in ALP (-16% to -19%) and aminotransferase levels without worsening pruritus.76 Diarrhoea was observed in about 25% of patients. An extended trial has been completed but the results have not yet been reported (NCT02135536).
Takeda G protein-coupled receptor 5 agonists
TGR5 (or GPBAR1) is a membrane-bound bile acid specific receptor expressed in biliary epithelial cells and various tissues, but not in hepatocytes. Experimental studies have shown that TGR5 activation increases cholangiocellular HCO3-/fluid secretion and negatively regulates hepatic inflammation.77 As a result, TGR5 agonists are potential candidates for the treatment of PBC despite expected off-target effects, such as inhibition of gallbladder contractibility, promotion of cell proliferation and suspected involvement in pruritus, which require consideration. However, no trials of pure TGR5 agonists are currently recruiting.
Norursodeoxycholic acid
NorUDCA is the C23 homologue of UDCA; norUDCA lacks 1 methylene group in its side chain, which confers a relative resistance to amidation. This side chain structure determines unique physiologic and pharmacologic properties including the ability to undergo cholehepatic shunting (instead of a full enterohepatic cycle) and to stimulate cholangiocyte secretion, which results in a bicarbonate-rich hypercholeresis leading to flushing of bile ducts and reinforcement of the protective biliary bicarbonate umbrella. In addition, antifibrotic and anti-inflammatory effects have been demonstrated. A large phase II, double-blind, randomised, placebo-controlled study evaluating the safety and efficacy of norUDCA for 12 weeks in patients with primary sclerosing cholangitis showed a significant biochemical improvement together with a good safety profile.78 Because of its properties, norUDCA warrants evaluation in patients with PBC, but no clinical trial is planned to date.
Ileal bile acid transporter inhibitors
The main goal of ileal bile acid transporter (IBAT) inhibitors is to improve pruritus by decreasing retained circulating bile acids that have been proposed as key players in the mechanisms of pruritus. Several compounds altering ileal reabsorption of bile acids (apical sodium-dependent bile acid transporter inhibitors) have been developed. Lopixibat was ineffective in reducing patient-reported pruritus scores (despite decreased serum bile acid levels) over a 12-week period.79 However, GSK2330672 showed efficacy on pruritus in a randomised, double-blind, placebo-controlled, cross-over, phase II study, without evidence of improved biochemical cholestasis over a 14-day period of time.80 Diarrhoea is the most common adverse effect and might limit the long-term use of IBAT inhibitors. A dose response study of GSK2330672 is in progress (NCT02966834).
Antifibrotic agents
Surprisingly, in contrast with other chronic liver diseases, very few clinical trials in PBC have tested agents targeting mainly fibrosis, such as inhibitors of integrin αVβ6 and lysyl oxidase homologue 2 (LOXL2). One exception is pentoxiphylline, a methylxantine derivative with potential antifibrotic properties, which has been tested. However, results are not currently available.
A dual inhibitor of NADPH oxidases (NOX) 1 and NOX4 (GKT13783) is being tested in PBC because of its anti-inflammatory and antifibrotic effects. Promising results of a phase II study have recently been reported and a phase III study is planned.81
Other speculative future developments
Real hope may also be based on manipulation of microbiome,82 mesenchymal stem cells (NCT01440309), and potentially anti-miR-506 strategies.15
Conclusions
Although UDCA will probably remain the standard of care for PBC for a long time to come, recent breakthroughs, including OCA and bezafibrate, mean that there are now effective second-line therapies for patients with incomplete response or intolerance to UDCA. Additional options, like new-generation FXR and PPAR agonists and bile acid reuptake inhibitors, should shortly complement the range of available treatments for PBC. The use of combination therapies in PBC is expected to increase significantly in the coming years, as a complete normalisation of the biochemical features of cholestasis becomes the next major challenge.
Conflict of interest
Dr. Corpechot reports receiving consulting fees from Intercept and Inventiva, grant support from Arrow and Intercept, and fees for teaching from GlaxoSmithKline; Dr. Poupon, receiving consulting fees from Intercept; Dr. Chazouillères, receiving grant support from Aptalis, fees for teaching from Mayoly Spindler, consulting fees from Genfit, and fees for teaching and consulting fees from Intercept; No other potential competing interest relevant to this article was reported.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.05.005.
Supplementary data
Supplementary material
References
- 1.EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitisJ Hepatol. 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 3.Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hubscher S. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568–1594. doi: 10.1136/gutjnl-2017-315259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harms MH, van Buuren HR, Corpechot C, Thorburn D, Janssen HLA, Lindor KD. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71:357–365. doi: 10.1016/j.jhep.2019.04.001. Epub 2019 Apr 11. [DOI] [PubMed] [Google Scholar]
- 5.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715–720. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Corpechot C, Abenavoli L, Rabahi N, Chretien Y, Andreani T, Johanet C. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871–877. doi: 10.1002/hep.22428. [DOI] [PubMed] [Google Scholar]
- 7.Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281–1287. doi: 10.1053/j.gastro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C. Baseline Ductopenia and Treatment Response Predict Long-Term Histological Progression in Primary Biliary Cirrhosis. Am J Gastroenterol. 2010;105:2186–2194. doi: 10.1038/ajg.2010.216. [DOI] [PubMed] [Google Scholar]
- 9.Corpechot C, Chazouilleres O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011;55:1361–1367. doi: 10.1016/j.jhep.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338–1349. doi: 10.1053/j.gastro.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Medina JF, Martinez A, Vazquez JJ, Prieto J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology. 1997;25:12–17. doi: 10.1002/hep.510250104. [DOI] [PubMed] [Google Scholar]
- 12.Prieto J, Garcia N, Marti-Climent JM, Penuelas I, Richter JA, Medina JF. Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology. 1999;117:167–172. doi: 10.1016/s0016-5085(99)70564-0. [DOI] [PubMed] [Google Scholar]
- 13.Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP. The biliary HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 14.Poupon R. Liver alkaline phosphatase: a missing link between choleresis and biliary inflammation. Hepatology. 2015;61:2080–2090. doi: 10.1002/hep.27715. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues PM, Perugorria MJ, Santos-Laso A, Bujanda L, Beuers U, Banales JM. Primary biliary cholangitis: A tale of epigenetically-induced secretory failure? J Hepatol. 2018;69:1371–1383. doi: 10.1016/j.jhep.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Erice O, Munoz-Garrido P, Vaquero J, Perugorria MJ, Fernandez-Barrena MG, Saez E. MicroRNA-506 promotes primary biliary cholangitis-like features in cholangiocytes and immune activation. Hepatology. 2018;67:1420–1440. doi: 10.1002/hep.29533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki M, Nakanuma Y. Stress-induced cellular responses and cell death mechanisms during inflammatory cholangiopathies. Clin Res Hepatol Gastroenterol. 2017;41:129–138. doi: 10.1016/j.clinre.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Increased expression of mitochondrial proteins associated with autophagy in biliary epithelial lesions in primary biliary cirrhosis. Liver Int. 2013;33:312–320. doi: 10.1111/liv.12049. [DOI] [PubMed] [Google Scholar]
- 19.Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Farkkila M. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549–558. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Slijepcevic D, van de Graaf SF. Bile Acid Uptake Transporters as Targets for Therapy. Dig Dis. 2017;35:251–258. doi: 10.1159/000450983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745–758. doi: 10.1016/j.jhep.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Parola M, Pinzani M. Pathophysiology of Organ and Tissue Fibrosis. Mol Aspects Med. 2019;65:1. doi: 10.1016/j.mam.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Bottcher K, Pinzani M. Pathophysiology of liver fibrosis and the methodological barriers to the development of anti-fibrogenic agents. Adv Drug Deliv Rev. 2017;121:3–8. doi: 10.1016/j.addr.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Poupon RE, Balkau B, Eschwege E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. N Engl J Med. 1991;324:1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 25.Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. N Engl J Med. 1994;330:1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 26.Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19:1149–1156. [PubMed] [Google Scholar]
- 27.Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–1290. doi: 10.1016/0016-5085(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 28.Combes B, Carithers RL, Jr., Maddrey WC, Lin D, McDonald MF, Wheeler DE. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22:759–766. [PubMed] [Google Scholar]
- 29.Van Hoogstraten HJ, De Smet MB, Renooij W, Breed JG, Engels LG, Den Ouden-Muller JW. A randomized trial in primary biliary cirrhosis comparing ursodeoxycholic acid in daily doses of either 10 mg/kg or 20 mg/kg. Dutch Multicentre PBC Study Group. Aliment Pharmacol Ther. 1998;12:965–971. doi: 10.1046/j.1365-2036.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- 30.Pares A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia-Plaza A. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA- Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32:561–566. doi: 10.1016/s0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 31.Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology. 2000;32:1196–1199. doi: 10.1053/jhep.2000.20240. [DOI] [PubMed] [Google Scholar]
- 32.Poupon RE, Lindor KD, Pares A, Chazouilleres O, Poupon R, Heathcote EJ. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39:12–16. doi: 10.1016/s0168-8278(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 33.Lindor KD, Jorgensen RA, Therneau TM, Malinchoc M, Dickson ER. Ursodeoxycholic acid delays the onset of esophageal varices in primary biliary cirrhosis. Mayo Clin Proc. 1997;72:1137–1140. doi: 10.4065/72.12.1137. [DOI] [PubMed] [Google Scholar]
- 34.Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–890. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 35.Shi J, Wu C, Lin Y, Chen YX, Zhu L, Xie WF. Long-term effects of mid-dose ursodeoxycholic acid in primary biliary cirrhosis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:1529–1538. doi: 10.1111/j.1572-0241.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- 36.Hempfling W, Dilger K, Beuers U. Systematic review: ursodeoxycholic acid--adverse effects and drug interactions. Aliment Pharmacol Ther. 2003;18:963–972. doi: 10.1046/j.1365-2036.2003.01792.x. [DOI] [PubMed] [Google Scholar]
- 37.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 38.Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67:1890–1902. doi: 10.1002/hep.29569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 40.Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trauner M, Nevens F, Shiffman ML, Drenth JPH, Bowlus CL, Vargas V. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol. 2019;4:445–453. doi: 10.1016/S2468-1253(19)30094-9. [DOI] [PubMed] [Google Scholar]
- 42.Bowlus C, Pockros P, Kremer A, Pares A, Forman L, Drenth J. Long-Term Obeticholic Acid (OCA) treatment associated with reversal or stabilization of fibrosis/cirrhosis in patients with Primary Biliary Cholangitis (PBC) J Hepatol. 2018;68:S111–S112. [Google Scholar]
- 43.Corpechot C, Chazouilleres O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med. 2018;378:2171–2181. doi: 10.1056/NEJMoa1714519. [DOI] [PubMed] [Google Scholar]
- 44.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 45.Post SM, Duez H, Gervois PP, Staels B, Kuipers F, Princen HM. Fibrates suppress bile acid synthesis via peroxisome proliferator-activated receptor-alpha-mediated downregulation of cholesterol 7alpha-hydroxylase and sterol 27-hydroxylase expression. Arterioscler Thromb Vasc Biol. 2001;21:1840–1845. doi: 10.1161/hq1101.098228. [DOI] [PubMed] [Google Scholar]
- 46.Chianale J, Vollrath V, Wielandt AM, Amigo L, Rigotti A, Nervi F. Fibrates induce mdr2 gene expression and biliary phospholipid secretion in the mouse. Biochem J. 1996;314:781–786. doi: 10.1042/bj3140781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwasaki S, Tsuda K, Ueta H, Aono R, Ono M, Saibara T. Bezafibrate may have a beneficial effect in pre-cirrhotic primary biliary cirrhosis. Hepatol Res. 1999;16:12–18. [Google Scholar]
- 48.Nakai S, Masaki T, Kurokohchi K, Deguchi A, Nishioka M. Combination therapy of bezafibrate and ursodeoxycholic acid in primary biliary cirrhosis: a preliminary study. Am J Gastroenterol. 2000;95:326–327. doi: 10.1111/j.1572-0241.2000.01667.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohira H, Sato Y, Ueno T, Sata M. Fenofibrate treatment in patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2147–2149. doi: 10.1111/j.1572-0241.2002.05944.x. [DOI] [PubMed] [Google Scholar]
- 50.Iwasaki S, Ohira H, Nishiguchi S, Zeniya M, Kaneko S, Onji M. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: A prospective, multicenter study. Hepatol Res. 2008;38:557–564. doi: 10.1111/j.1872-034X.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 51.Levy C, Peter JA, Nelson DR, Keach J, Petz J, Cabrera R. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2011;33:235–242. doi: 10.1111/j.1365-2036.2010.04512.x. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka A, Hirohara J, Nakanuma Y, Tsubouchi H, Takikawa H. Biochemical responses to bezafibrate improve long-term outcome in asymptomatic patients with primary biliary cirrhosis refractory to UDCA. J Gastroenterol. 2015;50:675–682. doi: 10.1007/s00535-014-0998-z. [DOI] [PubMed] [Google Scholar]
- 53.Reig A, Sese P, Pares A. Effects of Bezafibrate on Outcome and Pruritus in Primary Biliary Cholangitis With Suboptimal Ursodeoxycholic Acid Response. Am J Gastroenterol. 2018;113:49–55. doi: 10.1038/ajg.2017.287. [DOI] [PubMed] [Google Scholar]
- 54.Kremer AE, Le Cleac'h A, Lemoinne S, Wolf K, De Chaisemartin L, Chollet-Martin S. Antipruritic effect of bezafibrate and serum autotaxin measures in patients with primary biliary cholangitis. Gut. 2018 doi: 10.1136/gutjnl-2018-317426. [DOI] [PubMed] [Google Scholar]
- 55.Ting RD, Keech AC, Drury PL, Donoghoe MW, Hedley J, Jenkins AJ. Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: the FIELD Study. Diabetes Care. 2012;35:218–225. doi: 10.2337/dc11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corpechot C, Chazouilleres O, Lemoinne S, Rousseau A. Letter: reduction in projected mortality or need for liver transplantation associated with bezafibrate add-on in primary biliary cholangitis with incomplete UDCA response. Aliment Pharmacol Ther. 2019;49:236–238. doi: 10.1111/apt.15049. [DOI] [PubMed] [Google Scholar]
- 57.Leuschner M, Maier KP, Schlichting J, Strahl S, Herrmann G, Dahm HH. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 1999;117:918–925. doi: 10.1016/s0016-5085(99)70351-3. [DOI] [PubMed] [Google Scholar]
- 58.Rautiainen H, Karkkainen P, Karvonen AL, Nurmi H, Pikkarainen P, Nuutinen H. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: A three-year randomized trial. Hepatology. 2005;41:747–752. doi: 10.1002/hep.20646. [DOI] [PubMed] [Google Scholar]
- 59.Hirschfield G, Kupcinskas L, Ott P, Beuers U, Bergquist A, Farkkila M. Results of a randomised controlled trial of budesonide add-on therapy in patients with primary biliary cholangitis and an incomplete response to ursodeoxycholic acid. J Hepatol. 2018;68:S38. [Google Scholar]
- 60.Hempfling W, Grunhage F, Dilger K, Reichel C, Beuers U, Sauerbruch T. Pharmacokinetics and pharmacodynamic action of budesonide in early- and late-stage primary biliary cirrhosis. Hepatology. 2003;38:196–202. doi: 10.1053/jhep.2003.50266. [DOI] [PubMed] [Google Scholar]
- 61.Rabahi N, Chretien Y, Gaouar F, Wendum D, Serfaty L, Chazouilleres O. Triple therapy with ursodeoxycholic acid, budesonide and mycophenolate mofetil in patients with features of severe primary biliary cirrhosis not responding to ursodeoxycholic acid alone. Gastroenterol Clin Biol. 2010;34:283–287. doi: 10.1016/j.gcb.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Murillo Perez CF, Gulamhusein A, Corpechot C, van der Meer A, van Buuren H, Invernizzi P. Alkaline phosphatase normalization is associated with a decreased risk for liver transplantation and death in patients with primary biliary cholangitis. Hepatology. 2018;68:1085A. [Google Scholar]
- 63.Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, Chazouilleres O. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198–208. doi: 10.1002/hep.25599. [DOI] [PubMed] [Google Scholar]
- 64.Grigorian AY, Mardini HE, Corpechot C, Poupon R, Levy C. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: A meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:296–306. doi: 10.1016/j.clinre.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 65.Pratt DS. Hepatology Elsewhere. Hepatology. 2019;69:2698–2700. doi: 10.1002/hep.30517. Epub 2019 Mar 27. [DOI] [PubMed] [Google Scholar]
- 66.Dyson JK, Hirschfield GM, Adams DH, Beuers U, Mann DA, Lindor KD. Novel therapeutic targets in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. 2015;12:147–158. doi: 10.1038/nrgastro.2015.12. [DOI] [PubMed] [Google Scholar]
- 67.Tsuda M, Moritoki Y, Lian ZX, Zhang W, Yoshida K, Wakabayashi K. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55:512–521. doi: 10.1002/hep.24748. [DOI] [PubMed] [Google Scholar]
- 68.Myers RP, Swain MG, Lee SS, Shaheen AA, Burak KW. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013;108:933–941. doi: 10.1038/ajg.2013.51. [DOI] [PubMed] [Google Scholar]
- 69.Khanna A, Jopson L, Howel D, Bryant A, Blamire A, Newton JL. Rituximab Is Ineffective for Treatment of Fatigue in Primary Biliary Cholangitis: A Phase 2 Randomized Controlled Trial. Hepatology. 2018 doi: 10.1002/hep.30099. [DOI] [PubMed] [Google Scholar]
- 70.Hirschfield GM, Gershwin ME, Strauss R, Mayo MJ, Levy C, Zou B. Ustekinumab for patients with primary biliary cholangitis who have an inadequate response to ursodeoxycholic acid: A proof-of-concept study. Hepatology. 2016;64:189–199. doi: 10.1002/hep.28359. [DOI] [PubMed] [Google Scholar]
- 71.Jones D, Boudes PF, Swain MG, Bowlus CL, Galambos MR, Bacon BR. Seladelpar (MBX-8025), a selective PPAR-delta agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716–726. doi: 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 72.Bowlus C, Neff G, Aspinall R, Galambos MR, Goel A, Hirschfield G. Efficacy and Safety of Seladelpar, a Selective Peroxisome Proliferator-Activated Receptor Delta Agonist, in Primary Biliary Cholangitis: 52-Week Analysis of an Ongoing International, Randomized, Dose Ranging Phase 2 Study. Hepatology. 2018;68:1445A. [Google Scholar]
- 73.Schattenberg J, Pares A, Kowdley KV, Heneghan MA, Caldwell S, Pratt D. Elafibranor, a peroxisome proliferator-activted receptor alpha and delta agonist demonstrates favourable efficacy and safety in patients with primary biliary cholangitis and inadequate response to ursodeoxycholic acid treatment. J Hepatol. 2019;70:e128. (LBO-102) [Google Scholar]
- 74.Schramm C, Hirschfield G, Mason A, Wedemeyer H, Klickstein L, Neelakantham S. Early assessment of safety and efficacy of tropifexor, a potent non bile-acid FXR agonist, in patients with primary biliary cholangitis: An interim analysis of an ongoing phase 2 study. J Hepatol. 2018;68:S103. [Google Scholar]
- 75.Verbeke L, Nevens F, Laleman W. Steroidal or non-steroidal FXR agonists - Is that the question? J Hepatol. 2017;66:680–681. doi: 10.1016/j.jhep.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 76.Mayo MJ, Wigg AJ, Leggett BA, Arnold H, Thompson AJ, Weltman M. NGM282 for Treatment of Patients With Primary Biliary Cholangitis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Hepatol Commun. 2018;2:1037–1050. doi: 10.1002/hep4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beuers U, Trauner M, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015;62:S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 78.Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Farkkila M. norUrsodeoxycholic Acid Improves Cholestasis in Primary Sclerosing Cholangitis. J Hepatol. 2017;67:549–558. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Mayo M, Pockros P, Jones D, Bowlus C, Levy C, Patanwala I. A Randomized, Controlled, Phase 2 Study of Maralixibat in the Treatment of Itching Associated With Primary Biliary Cholangitis. Hepatol Commun. 2019;3:365–381. doi: 10.1002/hep4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Thompson D, Richards D. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–1123. doi: 10.1016/S0140-6736(17)30319-7. [DOI] [PubMed] [Google Scholar]
- 81.Dalekos GN, Invernizzi P, Nevens F, Van Vlierberghe H, Zigmond E, Andrade RJ. Efficacy of GKT831 in patients with primary biliary cholangitis and inadequate response to ursodeoxycholic acid: Interim efficacy results of a phase 2 clinical trial. J Hepatol. 2019;70:e1–e2. [Google Scholar]
- 82.Tang R, Wei Y, Li Y, Chen W, Chen H, Wang Q. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67:534–541. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material