Summary
In the era of the “sickest first” policy, patients with very high model for end-stage liver disease (MELD) scores have been increasingly admitted to the intensive care unit with the expectation that they will receive a liver transplant (LT) in the absence of improvement on supportive therapies. Such patients are often admitted in a context of acute-on-chronic liver failure with extrahepatic failures. Sequential assessment of scores or classification based on organ failures within the first days after admission help to stratify the risk of mortality in this population. Although the prognosis of severely ill cirrhotic patients has recently improved, transplant-free mortality remains high. LT is still the only curative treatment in this population. Yet, the increased relative scarcity of graft resource must be considered alongside the increased risk of losing a graft in the initial postoperative period when performing LT in “too sick to transplant” patients. Variables associated with poor immediate post-LT outcomes have been identified in large studies. Despite this, the performance of scores based on these variables is still insufficient. Consideration of a patient’s comorbidities and frailty is an appealing predictive approach in this population that has proven of great value in many other diseases. So far, local expertise remains the last safeguard to LT. Using this expertise, data are accumulating on favourable post-LT outcomes in very high MELD populations, particularly when LT is performed in a situation of stabilization/improvement of organ failures in selected candidates. The absence of “definitive” contraindications and the control of “dynamic” contraindications allow a “transplantation window” to be defined. This window must be identified swiftly after admission given the poor short-term survival of patients with very high MELD scores. In the absence of any prospect of LT, withdrawal of care could be discussed to ensure respect of patient life, dignity and wishes.
Keywords: liver transplantation, cirrhosis, acute-on-chronic liver failure, organ failure, acute decompensation, transplantation window
Introduction
Since the first experiment by Thomas E Starzl in the 1960s,1 liver transplants (LTs) have revolutionized the treatment of patients with severe liver disease, dramatically improving outcomes. Illustrating the success of the procedure, around 23,000 transplants were performed around the world in 2017.2 However, access to LT is limited. A stagnant pool of donor organs contrasts with an increasing number of candidates. In France, for instance, the overall number of newly listed patients increased by 24% between 2011 and 2017 with an average 2.4 transplant candidates per available graft.3 Regulation regarding the allocation system has led to grafts being offered to the patients with the highest risk of short-term mortality. As a consequence, severely ill patients with end-stage liver disease (ESLD), prioritized for LT, have been increasingly admitted to the intensive care unit (ICU).4 Outcomes of patients with ESLD in the ICU used to concern intensivists due to their outcomes compared to the general population.[5], [6] However, improved practices have led to an outstanding 30 point short-term survival gain between 2000 and 2010 in a liver-specific ICU.7 The reasons for such improvements are fourfold: better identification of candidates for ICU admission based on specific prognosis scores,[8], [9] better understanding of the pathophysiology of complications in chronic liver diseases, general improvement in ICU care,[10], [11] and development of specific management for patients with advanced liver disease. Indeed, in the last decades, there have been numerous medical improvements in the management of precipitating events and complications of ESLD. For instance, improvement in administration of antimicrobial therapy and bundle of care in sepsis resulted in better outcomes in both sepsis and septic shock.[12], [13], [14] Among others, the use of vasopressors, antibiotic prophylaxis and endoscopic management resulted in significant improvements in prognosis for patients admitted in the context of acute variceal bleeding.[15], [16], [17], [18] In the setting of hepatorenal syndrome (HRS), the combination of using albumin infusions, and vasopressors alongside clearer disease definitions has led to significant improvements in terms of outcome.[19], [20], [21], [22] Despite these improvements, LT remains the only life-saving procedure in patients that fail to recover adequate liver function. In order to illustrate the importance of “urgent” LT in this situation, the “share 35” policy has recently been implemented in the US to improve equity in access to LT, based on the comparable outcomes observed in patients with very high MELD scores without LT as in patients with fulminant hepatic failure (FHF).[23], [24], [25] With Share 35, patients with ESLD are now given the same geographic access to organs as patients with FHF, while the latter group is still granted higher allocation status in a given region.24 Several unmet medical and ethical issues have to be faced by clinicians in order to optimize management of patients with very high MELD scores from the perspective of LT, such as: How should these patients be categorized in the era of the dual concepts of acute-on-chronic liver failure (ACLF) and “mere” acute decompensation? Which prognosis assessment should be used? In the context of permanent scarcity of resource, how should the priority access to LT be managed in the most severely ill patients given the potential increased risk of post-LT mortality? Can upper limits of sickness be identified beyond which a patient should not be listed for an LT? How should we deal with patients already listed with less severe disease who are exposed to brutal deterioration of liver function and death due to restricted access to LT? Finally, how to identify and manage patients that are not candidates for LT and who do not recover adequate liver function?
We hereby offer to discuss all these important questions and review the literature in order to shed light on the management of patients with very high MELD scores.
Very high MELD and relationship to Acute-on-Chronic Liver Failure
Acute-on-chronic liver failure or "mere" acute decompensation?
Patients with very high MELD scores are often admitted in the context of acute decompensation (i.e. ascites, gastrointestinal bleeding, hepatic encephalopathy and/or acute bacterial infections). Distinctions have been made between “mere” acute decompensation and ACLF regarding their respective outcomes. “Mere” acute decompensation is associated with an acceptable 28-day and 3-month mortality (5% and 14% respectively)26 and is defined by either the absence of organ failures (OFs) or the presence of cerebral failure with a serum creatinine level < 1.5 mg/dl or the presence of 1 “non-kidney” OF with serum creatinine level < 1.5 mg/dl and no encephalopathy (Table 1). The median MELD of patients hospitalized in this setting is 16.27 Patients with very high MELD scores are more likely to be admitted in the context of ACLF. The World Gastroenterology Organization defined ACLF as a syndrome in patients with chronic liver disease with or without previously diagnosed cirrhosis which is characterized by acute hepatic decompensation resulting in liver failure (jaundice and prolongation of the international normalized ratio) and 1 or more extrahepatic OFs that is associated with increased mortality within a period of 28 days and up to 3 months from onset.28 By definition, 28-day and 3-month mortality in this setting is high (respectively around 35% and 50%).[26], [29] Three types of ACLF were defined according to absence (Type A) or presence (Type B) of cirrhosis and history of previous hepatic decompensation (Type C). The latter 2 are the most prevalent in western countries.28 Despite agreement on the definition, exact implications of concepts such as “acute” and “failure” remain debated among the different learned societies.[26], [30], [31], [32]
Table 1.
ACLF grading according to EASL-CLIF consortium.26
| ACLF grade | Definition |
|---|---|
| No ACLF | Absence of organ failure OR Presence of one “non-kidney” failure + creatinine < 1.5 mg/dl + absence of encephalopathy OR Cerebral failure + creatinine < 1.5 mg/dl |
| Grade 1 | Kidney failure OR Presence of one non-kidney failure + creatinine between 1.5 to 1.9 mg/dl and/or mild to moderate encephalopathy OR Cerebral failure + creatinine between 1.5 to 1.9 mg/dl |
| Grade 2 | Two organ failures |
| Grade 3 | Three organ failures or more |
Which prognostic score to use and how to use them?
In cases of ACLF, standard liver disease prognostic scores underestimate short-term mortality, which is more closely associated with the number of OFs than with liver failure per se.[33], [34], [35] It is well established that, in such patients often hospitalized in the ICU, ICU scores (simplified acute physiology score II, sequential organ failure assessment [SOFA], acute physiology and chronic health evaluation score [APACHE]) evaluating extrahepatic OFs are better predictors of mortality than the MELD and other scores.[33], [35], [36], [37]
The Chronic Liver Failure Consortium (CLIF-C) of the European Association for the Study of the Liver Disease (EASL) has led a European prospective multicentre (29 centres) study involving 1,343 patients hospitalized for acute deterioration of hepatocellular function in order to establish diagnostic criteria for ACLF and assess the natural history of the syndrome (CANONIC cohort). In this cohort, OF was defined by CLIF-SOFA26 (adapted from the SOFA)38 and then by the CLIF-C OF score.39 The authors proposed arbitrarily 6 thresholds (identical in the 2 scores) to define OFs according to the 6 organ systems included (liver, coagulation, kidney, brain, respiration, circulation) and a subsequent grading ranging from 0 (absence of ACLF) to 3 depending on the number of OFs (Table 1, Table 2). This classification identifies 3 populations with different prognoses at 28-days, illustrated by mortality rates of 22% 32% and 76.7% in patients with ACLF grade 1, 2, and 3, respectively.26 ACLF is a dynamic process in which the rapid evolution after the first days of medical care is associated with clinical outcome (Fig. 1). Identification of an early non-severe course (resolution of ACLF or ACLF grade 1 after day 3 to 7) is associated with low to moderate 28-day transplant-free mortality ranging from 6–18%. On the contrary, early severe course (ACLF grade 2 or 3 at day 3 to 7) is associated with a high 28-day transplant-free mortality ranging from 42–92%.29 Therefore, sequential evaluation during the first days of care is required in order to optimize prediction of outcome and subsequent medical management in this population. A score derived from CLIF-C OF, named CLIF-C ACLF, which additionally included age and log-transformed white-blood cell count was initially reported to have the best performance in predicting short-term mortality in this population. These results were not confirmed in a recent study of more than 800 patients with ACLF. In this work, CLIF-C ACLF failed to show better perfomance in prediction of 90 days mortality compared to MELD (AUROC 0.68 vs. 0.67, p=0.3).[39], [40] Moreover, its daily use seems less convenient than the evaluation of OF number by the CLIF-C OF. Lactate levels were independently associated with short-term mortality in critically ill patients with liver cirrhosis and were shown to significantly improve performance of CLIF-C ACLF. The modified called CLIF-C ACLFsLact score remains to be evaluated in an external cohort41 together with the numerous biomarkers that have recently been associated with outcomes in this population.[42], [43], [44], [45], [46], [47] Combining available scores with biomarkers could optimize prediction of events in this population with a high short-term mortality.
Table 2.
Organ failures as defined by CLIF-C OF score.39
| Organ failure | Definition |
|---|---|
| Liver | Bilirubin level ≥ 12 mg/dl |
| Kidney | Serum creatinine level ≥ 2.0 mg/dl or renal replacement therapy |
| Brain | Grade 3 or 4 hepatic encephalopathy⁎(WH scale) |
| Circulatory | Use of vasopressors |
| Respiratory | PaO2/FiO2 ≤ 200# OR SpO2/FiO2 ≤ 214# |
FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen; SpO2, pulse oximetric saturation; WH, West-Haven.
Patients submitted to mechanical ventilation (MV) due to HE and not due to a respiratory failure were considered as presenting a cerebral failure.
Other patients with MV were considered as presenting a respiratory failure.
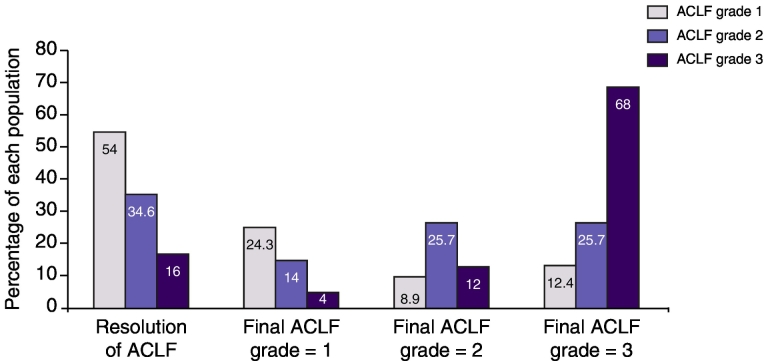
Fig. 1.
Histograms illustration of clinical course of ACLF depending on initial ACLF grade. Around 80% of patients with initial ACLF grade 1 will observe resolution of ACLF or will stabilized at grade 1. On the other side, only a small proportion (but non-null - 20%) of patients with initial ACLF grade 3 will observe final ACLF grade 1 or resolution of ACLF. ACLF, acute-on-chronic liver failure.
According to the North American Consortium for the Study of Liver Disease (NACSELD), ACLF syndrome is defined by 2 or more extrahepatic OFs among the following: circulatory failure defined by a shock, brain failure by grade III/IV hepatic encephalopathy, renal failure by requiring renal replacement therapy and respiratory failure by requiring mechanical ventilation.[32], [48] The main difference between this definition and EASL-CLIF consortium is the stringent definition of renal failure restricted to requirements of renal replacement therapy and the absence of liver-related variables (i.e. bilirubin and international normalized ratio). While 22.6% (303/1,343) of patients fulfilled the ACLF definition of the EASL-CLIF consortium at enrolment in the princeps European study,26 only 10% (264/2,675) fulfilled the NACSELD’s definition in a large study published recently.48 In the latter, including a large multinational cohort of infected and non-infected cirrhotic patients, NACSELD-ACLF was an independent prognostic factor of 30-day survival and the number of OFs (1, 2, 3 or 4) was also associated with 30-day survival (respectively around 85%, 73%, 53% and 12%). A substantial proportion of patients with very high MELD scores were excluded from the NACSELD-ACLF definition due to the stringent definition of renal failure. Moreover, excluding liver-related variables could include patients without very high MELD scores. We shall therefore hereafter focus on the patients with very high MELD scores, hospitalized in a context of ACLF according to the EASL-CLIF consortium classification as recommended in the 2018 published guidelines (the diagnosis and the grading of ACLF should be based on the assessment of organ function as defined by the CLIF-C OF score).49
According to these data, in daily practice, the prognosis of patients with very high MELD scores, hospitalized in the context of ACLF, can be assessed by the grade of ACLF on admission. Sequential evaluation at day 3 to day 7 appears critical in order to characterize the course of ACLF (severe vs. non-severe) and optimize medical management.
Type of organ failure, precipitating events and association with outcome
Prognosis by type of organ failure
Kidney failure (55.8% of patients) was the most common failure reported in the CANONIC cohort followed by liver failure (43.6% of patients), coagulation (27.7% of patients), brain (24.1% of patients), circulation (16.8% of patients) and the lungs (9.2% of patients). The impact of the type of failure on short-term outcome remains debated. Requirements for vasopressors and mechanical ventilation were identified as the failures associated with the worst outcomes in some studies[32], [48], [50] whereas this was not reported in a large recent international study.40 Liver failure, as defined by a total bilirubin ≥ 12 mg/dl in the CANONIC study, was an independent predictor of a severe life-threatening course of ACLF, suggesting a central role as a trigger or perpetuating factor of multi-organ failure.29
Do precipitating events have an impact on outcome?
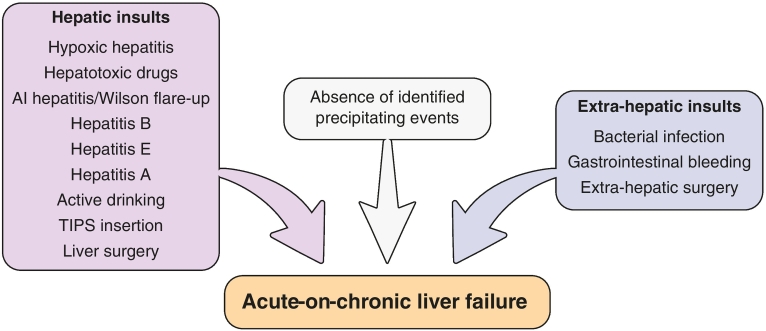
In the princeps study on ACLF, precipitating events were sepsis (33%), alcohol consumption (25%), gastrointestinal bleeding (13%) or others (8% transjugular portosystemic intrahepatic shunt insertion, surgery, viral hepatitis).26 Multiple precipitating events were reported in 13% of the population whereas no precipitating events were identified in around 44% of patients.26 The absence of a precipitating event was not associated with a different clinical course.[26], [51] An absence of precipitating events was thought to be linked with either the lack of sensitivity of diagnostic tests for bacterial infections, spontaneous resolution of events, or bacterial translocation episodes.[52], [53], [54], [55] The type and distribution of precipitating events varies according to the regions of the world. For instance, if viral hepatitis represented a minority of identified precipitating events in the CANONIC cohort, it represented more than 40% of events in a large Chinese study.56 The type of precipitating events was not related to 28-day mortality but could be related to 3-month mortality when divided into “hepatic” or “extrahepatic” events[26], [56] (Fig. 2). In a subset of the CANONIC database, bacterial infection as the trigger of ACLF was associated with poorer outcome than ACLF not triggered by sepsis, with a 3-month transplant-free mortality of 50.7% vs. 38.4%, p < 0.05. In the same study, infections with multidrug resistant bacteria that triggered ACLF and/or severe sepsis/septic shock at diagnosis were associated with worse prognosis.57 Therefore, according to the recent recommendations from EASL, potential precipitating factor(s), either hepatic (i.e. heavy alcohol intake, viral hepatitis, drug-induced liver injury, autoimmune hepatitis) and/or extrahepatic (i.e. infections, haemodynamic changes following haemorrhage, surgery) should be investigated. Early identification and treatment, particularly bacterial infections, are recommended.49
Fig. 2.
Illustration of most listed precipitating events leading to ACLF and classified as intrinsic (hepatic), extrinsic (extrahepatic) and non-identified ones. The latter ones are mainly sought to be related with bacterial translocation not documented due to a lack of sensitivity of bacterial tests. Distribution of these events varies significantly according to the region of the world. Association of type of events on outcomes is still a controversial issue that needs further exploration.
ACLF, acute-on-chronic liver failure.
Management and therapies
General management
In the population of patients with very high MELD scores, no specific treatment is available aside from antiviral therapy in patients with ACLF due to reactivation of HBV infection.[49], [55], [57] The management is mainly based on organ support together with the prevention and the treatment of associated complications. Such medical care requires a multidisciplinary approach that includes intensivists, specialists in infectious diseases, and a transplant team made up of hepatologists, surgeons and anaesthesiologists.59 Because of the severe prognosis particularly observed in ACLF grade 2 or 3 (corresponding to patients with very high MELD scores), patients should preferably be managed in the ICU of an LT centre as recommended by EASL guidelines (early referral of patients with ACLF to LT centres for immediate evaluation is recommended).[49], [57] Major improvements in prognosis of critically ill cirrhotic patients have been observed over the past decades.7 In parallel, outcomes of patients with ACLF have been shown to be similar to those of patients without liver disease who are admitted to the ICU with sepsis or with other critical conditions.60 Therefore, in our opinion, access to the ICU for this population should not be denied without strong evidence of futility of care assessed by a multidisciplinary evaluation.
Among the specific bundle of care in this population, a particular effort should be made to time initial resuscitation in order to control the extension of multi-organ failure. For instance, each hour delay in appropriate antimicrobial therapy is associated with significantly increased hospital mortality.[61], [62] Moreover, prophylactic strategies should be used to prevent bacterial infection, considering its deleterious influence on prognosis in the setting of ACLF triggered by non-infectious factors.57
General management of such patients should be made in accordance with recent guidelines and expert reviews on critically ill cirrhotic patients.[55], [59], [63], [64], [65] This review will then focus only on the few different alternative strategies available in this population.
Therapies
Liver transplantation
Who to transplant?
In the absence of effective alternative strategies, LT remains the unique curative treatment available in this population who has been granted priority access. Indeed, the MELD score was first proposed to predict the 3-month mortality in cirrhotic patients after transjugular portosystemic intrahepatic shunt insertion.66 Later on, this score was widely implemented in graft allocation scores to prioritize patients disclosing the highest risk of waiting list mortality, thus leading to the “sickest first” policy.[67], [68], [69], [70] The survival benefit conferred to recipients of LTs increases progressively with MELD score when compared to candidates on the LT waiting list. The lower threshold of MELD identifying patients who will benefit from LT is not well established (around 15) and could vary according to the aetiology of underlying liver disease.[71], [72] Nevertheless, the maximum benefit of LT is constantly observed in the sickest patients with the highest MELD scores (i.e. ≥ 40).[71], [72], [73] The MELD is currently capped at 40 while waiting list mortality continues to increase over this score, penalizing transplant candidates with a capped score.74 Despite the obvious theoretical benefit of this procedure, performing LT in patients with very high MELD scores hospitalized in the context of ACLF remains controversial. Indeed, some studies reported an increased mortality and morbidity after LT in recipients with very high MELD scores compared to populations with lower MELD scores[75], [76], [77] whereas others did not.[78], [79] In the context of a global organ shortage in LT, the concept of utility has therefore been opposed to the one of equity.80 This raises several unsolved issues linked to the question of results of LT in patients with very high MELD scores, identification of “too sick to transplant” patients and the associated upper limits of the “sickest first” policy.
In recent years, the notion of “futility” of LT has been used if post-transplant mortality was higher than waiting list mortality[81], [82] or in case of 3-month or in-hospital mortality after LT.[76], [83] However, authors of a recent review preferred to correct “futile” LT to “potentially inappropriate” LT, thus reducing the value judgment inherent to the term “futile”.80 This semantic change follows the adoption of these new terms by a multisociety statement that provided guidance for clinicians to prevent and manage disputes regarding patients with advanced critical illnesses.84 This change introduces an element of uncertainty in this area (i.e. “potentially”) when considering the fact that medicine is always evolving and upper limits always repelled.85
The terms of “potentially inappropriate LT” should therefore be preferred to “futile LT”. Identification of variables associated with post-LT outcomes in the setting of patients with very high MELD should help identify clinical situations where LT is potentially inappropriate.
Pretransplant variables vs. post-transplant outcomes
MELD alone does not show good enough performance in identifying patients with high rates of short-term post-LT mortality even in the most severe patients using uncapped MELD with scores > 40.[74], [86] Therefore, many authors have searched for recipient factors associated with 3-month mortality or in-hospital mortality. Thirteen variables were identified in a retrospective study from 21,673 patients in the United Network for Organ Sharing (UNOS) database81 leading to the development of the P-SOFT (for Preallocation Survival Outcomes Following Liver Transplantation) and SOFT scores (after inclusion of graft related variables) which show good performances in prediction of 3-month post-LT mortality with a c-statistic at 0.69 and 0.70 respectively. The BAR (BAlance of Risk) score was developed based on the 37,255 patients from the UNOS database after identification of 4 simple recipient variables (age, MELD, life support pretransplantation and retransplantation) and 2 donor-related variables associated with 3-month post-LT mortality.76 This score showed similar performance in predicting 3-month post-LT mortality (c-statistic of 0.7). The ACLF-score identified 5 recipient characteristics (age, gender, ESLD or hepatocellular carcinoma, recent infection and presence of ACLF) and 1 donor characteristic (gender) as independently associated with mortality 3 months after LT.87 This score also showed comparable predictive performance with a c-statistic test at 0.71.
Apart from age, none of these scores contain variables evaluating comorbidities. Through various diseases, comorbidities have been associated with outcome.[88], [89], [90], [91], [92] Screening of significant extrahepatic comorbid conditions is the cornerstone of the health assessment required before placement on a waiting list. The UCLA score was developed with the aim of identifying variables associated with 3-month or in-hospital mortality after LT in patients with very high MELD scores (≥ 40). Among the evaluated variables, authors chose the Charlson-comorbidity index (CCI)93 that had already been proven in a recalibrated form to be efficient in determining outcome in an LT population.94 Another score named the “cardiac risk score” was defined by the presence of at least 1 of the following variables: severe valvular disease, coronary artery disease with more than 70% stenosis or previous revascularization, history of myocardial infarction, history of ventricular and/or atrial arrhythmias, elevated pre-orthotopic LT troponin I (> 0.2 ng/ml), and/or new wall motion abnormalities on echocardiography. Indeed, cardiovascular disease was shown to be associated with short-term outcome, particularly in the first month following LT (mortality or cardiovascular events).[95], [96] MELD score, pretransplant septic shock, but also age-adjusted CCI and cardiac risk score were associated with outcomes using a multivariate analysis. In the final score named UCLA futility risk score (UCLA-FRS) an important weight was given to comorbidity including the cardiac risk index and the CCI: UCLA-FRS = 0.5 × (MELD score) + 5 × (1 = CCI ≥ 6; 0 = CCI < 6) + 4 × (1 = cardiac risk; 0 = no cardiac risk) + 3 × (1 = septic shock; 0 = no septic shock).97 In a recent study reporting results of LT in patients with OFs, this score seemed to be helpful in selecting candidates for LT when considering predicted and observed post-LT survival rates.98 Another study aimed to compare available scores in 2 independent cohorts of transplanted patients with high MELD scores (Swiss and UNOS cohort). Most prediction scores showed low positive predictive values for post-transplant mortality despite good specificity. Among these scores, the BAR score was the only score linearly associated with complications.99 The usefulness of this score, which was designed for the more critically ill patients, seems restricted to this population based on its performance in other cohorts100 (Table 3).
Table 3.
Available scores predicting early post-LT outcome and including recipient variables, adapted from77.
| Score | Recipient variables | Graft variables | C-stat 3-month post-LT mortality⁎ |
|---|---|---|---|
| P-SOFT# and SOFT79 |
Age, BMI, previous transplant, previous abdominal surgery albumin, dialysis prior to transplantation, intensive care unit pretransplant, admitted to hospital pretransplant, MELD score, life support pretransplant, encephalopathy, portal vein thrombosis, ascites pretransplant, portal bleed 48 h pretransplant | Age, cause of death, creatinine, allocation, CIT |
0.69 and 0.70 |
| BAR score73 | MELD score, previous LT, life support, recipient age | Donor age, CIT | 0.70 |
| UCLA-FRS94 | MELD score, septic shock, cardiac risk, age-adjusted Charlson comorbidity index | None | 0.75 |
| ACLF-Score84 | Gender, ESLD or HCC, ongoing infection, age, presence of ACLF | Gender | 0.71 |
SOFT, survival outcome following liver transplantation score; P-SOFT, preallocation-SOFT; BMI, body mass index; MELD, model for end-stage liver disease; CIT, cold-ischemia time; BAR, balance of risk; UCLA-FRS, University of California, Los Angeles-futility risk score; ACLF, acute-on-chronic liver failure, ESLD, end-stage liver disease, HCC, hepatocellular carcinoma
C-stat for 3-month and/or in-hospital mortality for the UCLA-FRS score.
P-SOFT includes only recipient variables whereas SOFT score includes recipient and graft variables.
An emerging concept in hepatology is frailty. Frailty corresponds to a validated geriatric construct of increased vulnerability to physiologic stressors. Frailty represents the conditions in a given patient that are unlikely to reverse after liver function returns or will take so long to reverse that the patient will be highly vulnerable to postoperative complications.[101], [102] Many tools assessing frailty have been proposed and evaluated in ESLD, particularly in patients awaiting LT: Fried frailty index, short physical performance battery,[102], [103] 6 minute walk test,104 activity of daily living.[105], [106] A Karnofsky performance status-based score has recently been proposed for the prediction of death after hospital discharge in cirrhosis.107 The subgroup of patients with low MELD scores and high frailty may derive the greatest benefit from implementation of these measures.102 However, functional tests evaluating frailty could be not enough discriminant in the homogeneous population of very high MELD patients. Moreover, it may not be possible to perform such tests because of patients’ clinical condition. An interesting approach will be the use of alternative tests to evaluate frailty, which are better adapted for an ICU population and do not require functional assessment.[108], [109] Evaluation of surrogate markers of frailty, such as sarcopenia, which can be assessed by imaging, represent an alternative option. When associated with MELD, CT-scan assessed sarcopenia has been demonstrated to be useful in the identification of patients with the highest risk of mortality on waiting lists.110 Moreover, sarcopenia was associated with post-LT mortality independently of the MELD score.111 These results were confirmed recently with different thresholds for women and men.112 Fat-free muscle mass assessed by MRI in decompensated patients was associated with mortality and also with the development of ACLF 113. However, data are lacking regarding the impact of sarcopenia on post-transplant outcome in patients with very high MELD scores in the context of ACLF. Sarcopenia is an evolving process and evaluation should be readily performed. In this view, assessment of sarcopenia by ultrasonography seems to be an easy and reliable option, but it is yet to be evaluated in cirrhotic patients.114
At the present time, identification of potentially inappropriate LT candidates should not be based solely on available scores considering their statistical performance. However, a combination of scores, as well as evaluation of frailty, could improve the clinical management of patients with very high MELD scores who are candidates for LT.
What is the outcome of ACLF transplanted patients?
Data on outcomes in patients with ACLF treated with liver LT are less scarce. Post-LT 3-month survival of recipients is about 80–90% and much higher than that anticipated if patients were not transplanted.[29], [50], [87], [98], [115], [116], [117] Indeed, in the CANONIC study, patients transplanted with an ACLF grade 2 and 3 between day 3 and day 7 (n = 21) had a better 6-month survival than the non-transplanted patients (80.9 vs. 10%, p < 0.001).29 Similarly, in our reported experience, the 73 patients transplanted with ACLF grade 3 at the time of LT had better 1-year survival than matched controls (83.9 vs. 7.9%, p < 0.0001).98 In a very large cohort study from the UNOS, performed in more than 11,000 patients transplanted with 1 or more OFs, a good 1-year survival ranging from 88% (1 OF) to 80% (5/6 OFs) was reported. Moreover, recipient’s long-term survival (5-year) was acceptable, ranging from 65% to 74% with similar results for liver graft survival.116 Regarding the impact of the type of OFs on post-LT outcomes, there was a lower post-LT survival among intubated patients or patients with circulatory failure than for other types of OF (1-year patient survival 79–81% vs. 84–87%).116 Levesque et al. recently reported a worse outcome following LT for patients with ACLF grade 3 compared to our cohort (43.3% survival at 1 year).87 The main difference in patient characteristics between this study and our collaborative work was the incidence of respiratory failure between the 2 groups of patients with ALCF grade 3 (76.7 vs. 15.8%).98 Together, these data suggest that severe and uncontrolled respiratory failure may be viewed as a contraindication for LT. This is in line with a recent report that identified severe acute respiratory distress syndrome as an independent factor associated with post-transplant mortality.118 Improvement or stabilization in OFs in the immediate pretransplant period has been associated with favourable post-LT outcomes in patients with ACLF, suggesting that the evolution of OFs should at least be controlled, if not improved, before considering an LT.[98], [115]
Another important point to consider is the timeframe of LT. In most studies reporting favourable post-LT outcomes in these severely ill patients, an “early” LT has been performed (time between placement on the waiting list and LT ranging from 3–9 days).[98], [116], [119] This very short timeframe is required because of the natural history of these patients. Indeed, around 50% of them will die in the first 10 days.116 A significant proportion of patients are not placed on the waiting list before acute deterioration of their condition. As an example, in our study, 45/73 patients transplanted with ACLF grade 3 were placed on waiting lists during the immediate pre-LT hospitalization (median time to LT: 8 days).98 Therefore, pretransplant evaluation must be performed in a timely fashion, precluding a number of clinical tests usually required in LT programmes. To our knowledge, the potential impact on post-LT outcomes of this “truncated” evaluation has yet to be studied extensively in patients with very high MELD scores.50 Such a “truncated” evaluation, involving the performance of only a few necessary screening tests, could lead to potential contraindications for LT (active neoplasia, severe alcohol dependence, or still significant coronary artery disease) being overlooked. In addition to the potential impact on outcomes, this raises some degree of unfairness with regard to less severe patients undergoing the recommended exhaustive evaluation (routinely responsible for not listing patients after the discovery of a contraindication).117 There is an urgent need to evaluate the various international LT centre practices in this setting. A prospective study validating a standardized assessment for patients with very high MELD scores, who have not yet been listed, should be put forward in order to optimize selection of candidates and decrease inequity.
While LT seems to confer favourable survival outcomes in these very sick patients, it is associated with longer total stay in both the ICU and the hospital. This also leads to a high rate of complications observed in this population, who therefore require attentive screening for complications, particularly of an infectious nature.[87], [98], [115], [117]
The current knowledge suggests that transplant outcomes in selected patients with ACLF could be favourable, even in patients with multi-organ failure at the time of LT. However, in order to optimize post-LT outcomes, LT should be performed in a timely manner after admission, within a window of improvement or at least stabilization of OFs.
When to transplant? The concept of a “transplantation window”
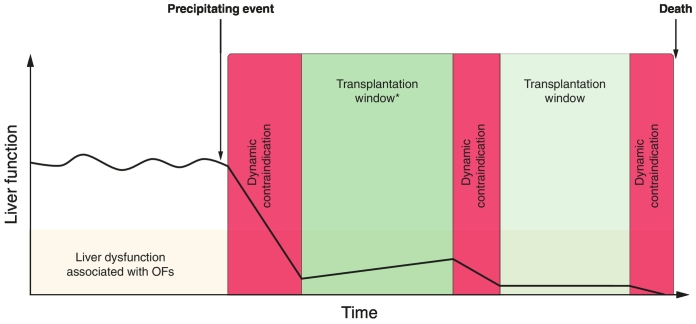
In order to identify the transplantation window, 2 classes of contraindications have to be separated. First, the “definitive” contraindications responsible for an immediate and irreversible arrest in the LT process at the local level. Some contraindications are widely accepted and might be illustrated by metastatic solid neoplasia, severe cardiac or pulmonary insufficiency (not related to liver disease), or even pre-hospitalization bedridden states. However, these “definitive” contraindications are definitive at a specific time in a specific transplant centre as their appreciation varies between LT centres depending on local expertise and the potential change over time within each centre.117 Second, the “dynamic” contraindications are responsible for a short temporary inability to perform liver transplantation. These “dynamic” contraindications are represented by numbers of diseases or failures that could evolve positively after appropriate therapeutic management: uncontrolled bacterial infection, active fungal infection, active gastrointestinal bleeding, severe acute respiratory distress syndrome, severe haemodynamic instability, severe coagulopathy. The “dynamic” contraindications are often represented by precipitating events of ACLF syndrome.[26], [56] Occurrence and resolution (or control) of “dynamic” contraindications in the context of stabilization or improvement of OFs define the “transplantation window”98 (Fig. 3). As discussed earlier, the optimal transplantation window should be identified as soon as possible after the admission of patients with very high MELD scores, considering their poor expected outcome and the risk of them developing adverse events (other “dynamic” contraindications in a short timeframe).[29], [120] Due to the lack of data in this field, the exact timeframes within which LT is contraindicated by “dynamic” contraindications are often transplant centre and local expertise dependant.
Fig. 3.
Illustration of "transplantation window" and "dynamic contraindication" relationship. Y axis illustrates “liver function” and X axis “time”. In this scenario, the onset of a precipitating event (bacterial pneumonia for example) leads to a rapid deterioration of liver function and ACLF in a patient with chronic liver disease. The “dynamic” contraindications (red boxes) illustrate a timeframe in which LT should not be performed due to uncontrolled clinical condition (e.g. severe acute respiratory syndrome with hemodynamic instability). After their resolution (or at least control – depending on local expertise) “dynamic” contraindications are separated by transplantation windows (green boxes) if LT is indicated. In these (often) short elapsed time in patient who were not already listed health assessment should be performed in order to exclude “definitive” contraindication to LT (e.g. metastatic neoplasia, severe cardiac insufficiency). If, the opportunity to transplant is not taken during the firsts transplantation windows, other events (e.g. other bacterial infection such as ventilation associated pneumonia, GI bleeding) can occur and lead patient to death. ACLF, acute-on-chronic liver failure; LT, liver transplantation; GI, gastrointestinal.
The concept of a transplantation window for LT in a patient without a “definitive” contraindication is based on the absence of “dynamic contraindications” that allow for LTs to be performed in a context of stabilization or improvement of OFs. The transplantation window should be rapidly identified following admission in order to optimize post-LT outcomes.
What to do in patients who are not transplanted because of recovery of liver function?
Studies reporting outcomes in cirrhotic patients after ICU discharge are scarce. However, after recovery from critical illness, cirrhotic patients are at higher risk of 1-year mortality (between 27.5% to 68%) compared to the general population.[121], [122], [123], [124] Predictors associated with 1-year mortality after ICU discharge are not clearly reported but could be related to the number and type of OFs that patients experienced in the ICU, as well as the severity of liver disease at admission to and discharge from the ICU.123 There are currently no reliable tools that identify populations with a very low risk of mortality after initial effective ICU care and discharge from the ICU. Therefore, such patients should be referred to liver transplant centres during the same hospitalization or closely thereafter, in order to be evaluated for LT.
Even in cases of recovery of liver function, patients with very high MELD scores admitted in the context of ACLF should be referred to LT centres as soon as possible, considering the risk of events at 1-year in this population.
Is there a place for extracorporeal liver support as bridging therapy to LT?
Modalities that can serve as a “bridge” to LT or that can confer sufficient clinical improvement to enable patients to be discharged from the ICU/hospital can positively impact outcomes in patients with very high MELD scores. These modalities aim to replace the functions of the failing liver, allowing hepatic recovery or stabilizing clinical state to enable transplantation.[55], [59] The key concepts of such therapies are to remove harmful toxins, support the liver for spontaneous regeneration and to reduce the ongoing inflammatory injury.59 Based on albumin dialysis, the molecular adsorbent recirculating system (MARS®) and plasma separation and absorption system (Prometheus®) are the most studied devices. MARS therapy has been reported to improve bilirubin levels, hepatic encephalopathy, haemodynamic parameters and kidney function in patients with decompensated cirrhosis[125], [126] without improving survival. In the RELIEF trial, including patients with ACLF, no improvement in transplant-free survival at 28 and 90 days was observed.127 In the Helios study, despite a well-tolerated treatment, Prometheus therapy was not reported to improve short-term survival in patients with ACLF.128 Of note, these results have to be interpreted with caution due to the heterogeneous population of patients with decompensated cirrhosis associated with different degrees of OFs and different definitions of ACLF.58 Results of a large prospective multicentre study evaluating a bioartificial device (extracorporeal cellular therapy) in patients with severe alcoholic hepatitis showed that it conferred no overall survival benefit compared to standard of care. However, among other limitations, patients were not stratified according to response to medical treatment and definitive conclusions on the efficacy of this device cannot, as of this date, be drawn.129 A second study in younger patients with lower MELD scores is therefore ongoing (NCT02612428).
Currently, 2 trials involving extracorporeal liver support are recruiting in the setting of ALCF. The first is evaluating the efficacy of high-volume plasma exchange and G-CSF vs. G-CSF alone in patients with ACLF (Clinicaltrials.gov identifier: NCT03162419). The second is a multicentre, randomized, controlled, study evaluating the safety and performance of a new liver support device called DIALIVE in 24 patients with ACLF vs. standard of care (NCT03065699).
Based on the results of available RCTs, EASL guidelines state that extracorporeal liver support systems do not improve survival of patients with ACLF and should therefore not be recommended for this indication.49
Other medical treatment
Among the medical treatments offered in this setting, G-CSF seems to be the most promising treatment, with accumulating evidence of its potential efficacy, albeit with only a small number of patients treated to date. G-CSF promotes mobilization of hematopoietic stem cells and induces proliferation of hepatic progenitor cells in animal models of liver failure but also in human alcoholic hepatitis.[130], [131] A few small-randomized clinical trials have demonstrated not only improvement in liver function with G-CSF but also significant survival benefit compared with standard medical therapy for ACLF.[132], [133], [134], [135]
Nonetheless, at this time, there is insufficient evidence for such therapies to be recommended in patients with very high MELD scores. This decision is essentially performed according to local expertise and beliefs. On this specific point EASL guidelines state that despite promising results, the administration of G-CSF cannot be recommended at present.49
What management to offer patients who do not receive a transplant given either their poor predicted survival or failure to receive a transplant within the target window?
The survival probability of patients with very high MELD scores is efficiently calculated by the available scores of ACLF (ACLF grade or CLIF-C ACLF).29 In the absence of alternative treatments for LT, due to the severe course observed in this population without LT, withdrawal of care could be a reasonable option in some patients presenting with a “definitive” contraindication. In the CANONIC cohort, sequential calculation of the scores was reported to identify a group of patients with the most severe outcome, with a 100% transplant-free mortality.29 These patients were identified as those with ≥ 4 OFs and/or CLIF-C ACLF ≥ 64 between day 3 and day 7. Based on these results, an algorithmic approach has proposed withdrawal of care to these patients.29 A retrospective analysis performed in the King’s College cohort7 using these thresholds reported that 10% of the patients survived at 90 days.136 This highlights that prediction scores perform differently in different cohorts and that medical management related to algorithm-based scores cannot be adopted widely without caution. It is important to recognize that any decision regarding withdrawal is irrevocable and small survival probabilities can be perceived differently by caregivers and patient’s families. Balancing these aspects while maintaining patient life, dignity, and wishes is fraught with statistical and ethical difficulties, and a single score is an unlikely final referee.136 Regarding the decision to withdraw care, data from the literature, as well as data from local experience should be considered, alongside the potential for improvement in OFs and qualitative factors (frailty and patient’s wishes).[136], [137], [138], [139] Finally, in our opinion, these scores are useful in identifying situations in which it could be reasonable to initiate discussion around withdrawal of care.
The EASL guidelines state that withdrawal of ongoing intensive care support can be suggested in patients who are not candidates for LT and who have 4 or more OFs after 1 week of adequate intensive treatment.49
Accurate identification of variables associated with mortality in patients with cirrhosis and the permanent scarcity of liver grafts has led to LT being recommended to an increasing number of severely ill patients. As a consequence, physicians in LT centres routinely face medical, surgical and ethical challenges. Nonetheless, data are accumulating on the pretransplant management of such patients and refined disease assessments based on variables such as clinical course, and the determination of risk factors for poor outcome after LT, may be made and help define a “too sick for transplant” upper-limit. However, while awaiting devices or treatment that will delay (or avoid) LT, there is an urgent need, based on robust data, to offer a consensual definition of “definitive” contraindications and the “transplantation window”.
Acknowledgements
We would like to acknowledge both Lille and Paul-Brousse Liver, ICU and Surgical teams for their help in the daily management of very high MELD patients.
References
- 1.Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. HOMOTRANSPLANTATION OF THE LIVER IN HUMANS. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 2.Global Observatory on Donation and Transplantation n.d.
- 3.Rapport médical et scientifique de l’Agence de la biomédecine 2017 n.d.
- 4.McPhail MJW, Parrott F, Wendon JA, Harrison DA, Rowan KA, Bernal W. Incidence and Outcomes for Patients With Cirrhosis Admitted to the United Kingdom Critical Care Units. Crit Care Med. 2018;46:705–712. doi: 10.1097/CCM.0000000000002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 6.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016–1020. doi: 10.1378/chest.124.3.1016. [DOI] [PubMed] [Google Scholar]
- 7.McPhail MJW, Shawcross DL, Abeles RD, Chang A, Patel V, Lee G-H. Increased Survival for Patients With Cirrhosis and Organ Failure in Liver Intensive Care and Validation of the Chronic Liver Failure-Sequential Organ Failure Scoring System. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015;13:1353–1360.e8. doi: 10.1016/j.cgh.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman JE, Wagner DP, Seneff MG, Becker RB, Sun X, Knaus WA. Intensive care unit admissions with cirrhosis: risk-stratifying patient groups and predicting individual survival. Hepatol Baltim Md. 1996;23:1393–1401. doi: 10.1002/hep.510230615. [DOI] [PubMed] [Google Scholar]
- 9.Tsai M-H, Chen Y-C, Ho Y-P, Fang J-T, Lien J-M, Chiu C-T. Organ system failure scoring system can predict hospital mortality in critically ill cirrhotic patients. J Clin Gastroenterol. 2003;37:251–257. doi: 10.1097/00004836-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 11.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 12.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 14.Galbois A, Aegerter P, Martel-Samb P, Housset C, Thabut D, Offenstadt G. Improved prognosis of septic shock in patients with cirrhosis: a multicenter study*. Crit Care Med. 2014;42:1666–1675. doi: 10.1097/CCM.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 15.de Franchis R, Baveno V Faculty Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 16.de Franchis R, Baveno VI Faculty Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatol Baltim Md. 2004;40:652–659. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- 18.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 19.Hadengue A, Gadano A, Moreau R, Giostra E, Durand F, Valla D. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol. 1998;29:565–570. doi: 10.1016/s0168-8278(98)80151-7. [DOI] [PubMed] [Google Scholar]
- 20.Uriz J, Ginès P, Cárdenas A, Sort P, Jiménez W, Salmerón JM. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol. 2000;33:43–48. doi: 10.1016/s0168-8278(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 21.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 22.Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatol Baltim Md. 2016;63:983–992. doi: 10.1002/hep.28396. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Schaubel DE, Gong Q, Guidinger M, Merion RM. End-stage liver disease candidates at the highest model for end-stage liver disease scores have higher wait-list mortality than status-1A candidates. Hepatol Baltim Md. 2012;55:192–198. doi: 10.1002/hep.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong AJ, Goel A, Mannalithara A, Kim WR. Improved posttransplant mortality after share 35 for liver transplantation. Hepatol Baltim Md. 2018;67:273–281. doi: 10.1002/hep.29301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murken DR, Peng AW, Aufhauser DD, Abt PL, Goldberg DS, Levine MH. Same policy, different impact: Center-level effects of share 35 liver allocation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2017;23:741–750. doi: 10.1002/lt.24769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437.e1-9. [DOI] [PubMed] [Google Scholar]
- 27.Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin H-C. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4–10. doi: 10.1053/j.gastro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatol Baltim Md. 2015;62:243–252. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 30.Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 32.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatol Baltim Md. 2014;60:250–256. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levesque E, Hoti E, Azoulay D, Ichaï P, Habouchi H, Castaing D. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56:95–102. doi: 10.1016/j.jhep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Das V, Boelle P-Y, Galbois A, Guidet B, Maury E, Carbonell N. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010;38:2108–2116. doi: 10.1097/CCM.0b013e3181f3dea9. [DOI] [PubMed] [Google Scholar]
- 35.Saliba F, Ichai P, Levesque E, Samuel D. Cirrhotic patients in the ICU: prognostic markers and outcome. Curr Opin Crit Care. 2013;19:154–160. doi: 10.1097/MCC.0b013e32835f0c17. [DOI] [PubMed] [Google Scholar]
- 36.Wehler M, Kokoska J, Reulbach U, Hahn EG, Strauss R. Short-term prognosis in critically ill patients with cirrhosis assessed by prognostic scoring systems. Hepatol Baltim Md. 2001;34:255–261. doi: 10.1053/jhep.2001.26522. [DOI] [PubMed] [Google Scholar]
- 37.Cholongitas E, Senzolo M, Patch D, Kwong K, Nikolopoulou V, Leandro G. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883–893. doi: 10.1111/j.1365-2036.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- 38.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 39.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Karvellas CJ, Garcia-Lopez E, Fernandez J, Saliba F, Sy E, Jalan R. Dynamic Prognostication in Critically Ill Cirrhotic Patients With Multiorgan Failure in ICUs in Europe and North America: A Multicenter Analysis. Crit Care Med. 2018 doi: 10.1097/CCM.0000000000003369. [DOI] [PubMed] [Google Scholar]
- 41.Drolz A, Horvatits T, Rutter K, Landahl F, Roedl K, Meersseman P. Lactate improves prediction of short-term mortality in critically ill cirrhosis patients: a multinational study. Hepatol Baltim Md. 2018 doi: 10.1002/hep.30151. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Margáin A, Pohlmann A, Ryan P, Schierwagen R, Chi-Cervera LA, Jansen C. Fibroblast growth factor 21 is an early predictor of acute-on-chronic liver failure in critically ill patients with cirrhosis. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2018;24:595–605. doi: 10.1002/lt.25041. [DOI] [PubMed] [Google Scholar]
- 43.AJC Kerbert, Verspaget HW, Navarro ÀA, Jalan R, Solà E, Benten D. Copeptin in acute decompensation of liver cirrhosis: relationship with acute-on-chronic liver failure and short-term survival. Crit Care Lond Engl. 2017;21 doi: 10.1186/s13054-017-1894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macdonald S, Andreola F, Bachtiger P, Amoros A, Pavesi M, Mookerjee R. Cell death markers in patients with cirrhosis and acute decompensation. Hepatol Baltim Md. 2018;67:989–1002. doi: 10.1002/hep.29581. [DOI] [PubMed] [Google Scholar]
- 45.Mookerjee RP. Prognosis and Biomarkers in Acute-on-Chronic Liver Failure. Semin Liver Dis. 2016;36:127–132. doi: 10.1055/s-0036-1583200. [DOI] [PubMed] [Google Scholar]
- 46.Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatol Baltim Md. 2007;45:62–71. doi: 10.1002/hep.21491. [DOI] [PubMed] [Google Scholar]
- 47.Ariza X, Graupera I, Coll M, Solà E, Barreto R, García E. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol. 2016;65:57–65. doi: 10.1016/j.jhep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 48.O’Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatol Baltim Md. 2018;67:2367–2374. doi: 10.1002/hep.29773. [DOI] [PubMed] [Google Scholar]
- 49.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Finkenstedt A, Nachbaur K, Zoller H, Joannidis M, Pratschke J, Graziadei IW. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2013;19:879–886. doi: 10.1002/lt.23678. [DOI] [PubMed] [Google Scholar]
- 51.Moreau R, Jalan R, Arroyo V. Acute-on-Chronic Liver Failure: Recent Concepts. J Clin Exp Hepatol. 2015;5:81–85. doi: 10.1016/j.jceh.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jalan R, Stadlbauer V, Sen S, Cheshire L, Chang Y-M, Mookerjee RP. Role of predisposition, injury, response and organ failure in the prognosis of patients with acute-on-chronic liver failure: a prospective cohort study. Crit Care Lond Engl. 2012;16:R227. doi: 10.1186/cc11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int Off J Int Assoc Study Liver. 2013;33:31–39. doi: 10.1111/liv.12021. [DOI] [PubMed] [Google Scholar]
- 54.Bajaj JS, O’Leary JG, Wong F, Reddy KR, Kamath PS. Bacterial infections in end-stage liver disease: current challenges and future directions. Gut. 2012;61:1219–1225. doi: 10.1136/gutjnl-2012-302339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet Lond Engl. 2015;386:1576–1587. doi: 10.1016/S0140-6736(15)00309-8. [DOI] [PubMed] [Google Scholar]
- 56.Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatol Baltim Md. 2015;62:232–242. doi: 10.1002/hep.27795. [DOI] [PubMed] [Google Scholar]
- 57.Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870–1880. doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
- 58.Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–553. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarin SK, Choudhury A. Management of acute-on-chronic liver failure: an algorithmic approach. Hepatol Int. 2018 doi: 10.1007/s12072-018-9887-5. [DOI] [PubMed] [Google Scholar]
- 60.Meersseman P, Langouche L, du Plessis J, Korf H, Mekeirele M, Laleman W. The intensive care unit course and outcome in acute-on-chronic liver failure are comparable to other populations. J Hepatol. 2018;69:803–809. doi: 10.1016/j.jhep.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 61.Arabi YM, Dara SI, Memish Z, Al Abdulkareem A, Tamim HM, Al-Shirawi N. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatol Baltim Md. 2012;56:2305–2315. doi: 10.1002/hep.25931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karvellas CJ, Abraldes JG, Arabi YM, Kumar A, Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015;41:747–757. doi: 10.1111/apt.13135. [DOI] [PubMed] [Google Scholar]
- 63.Nadim MK, Durand F, Kellum JA, Levitsky J, O’Leary JG, Karvellas CJ. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J Hepatol. 2016;64:717–735. doi: 10.1016/j.jhep.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 64.Fuhrmann V, Whitehouse T, Wendon J. The ten tips to manage critically ill patients with acute-on-chronic liver failure. Intensive Care Med. 2018 doi: 10.1007/s00134-018-5078-z. [DOI] [PubMed] [Google Scholar]
- 65.Olson JC, Karvellas CJ. Critical care management of the patient with cirrhosis awaiting liver transplant in the intensive care unit. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2017;23:1465–1476. doi: 10.1002/lt.24815. [DOI] [PubMed] [Google Scholar]
- 66.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatol Baltim Md. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 67.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL. A model to predict survival in patients with end-stage liver disease. Hepatol Baltim Md. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 68.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK. MELD and PELD: application of survival models to liver allocation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2001;7:567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 69.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2004;10:7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 70.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 71.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 72.Lucey MR, Schaubel DE, Guidinger MK, Tome S, Merion RM. Effect of alcoholic liver disease and hepatitis C infection on waiting list and posttransplant mortality and transplant survival benefit. Hepatol Baltim Md. 2009;50:400–406. doi: 10.1002/hep.23007. [DOI] [PubMed] [Google Scholar]
- 73.Luo X, Leanza J, Massie AB, Garonzik-Wang JM, Haugen CE, Gentry SE. MELD as a metric for survival benefit of liver transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2018;18:1231–1237. doi: 10.1111/ajt.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nadim MK, DiNorcia J, Ji L, Groshen S, Levitsky J, Sung RS. Inequity in organ allocation for patients awaiting liver transplantation: Rationale for uncapping the model for end-stage liver disease. J Hepatol. 2017;67:517–525. doi: 10.1016/j.jhep.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weismüller TJ, Fikatas P, Schmidt J, Barreiros AP, Otto G, Beckebaum S. Multicentric evaluation of model for end-stage liver disease-based allocation and survival after liver transplantation in Germany--limitations of the ’sickest first’-concept. Transpl Int Off J Eur Soc Organ Transplant. 2011;24:91–99. doi: 10.1111/j.1432-2277.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- 76.Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745–753. doi: 10.1097/SLA.0b013e3182365081. discussion 753. [DOI] [PubMed] [Google Scholar]
- 77.Bittermann T, Makar G, Goldberg DS. Early post-transplant survival: Interaction of MELD score and hospitalization status. J Hepatol. 2015;63:601–608. doi: 10.1016/j.jhep.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desai NM, Mange KC, Crawford MD, Abt PL, Frank AM, Markmann JW. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99–106. doi: 10.1097/01.TP.0000101009.91516.FC. [DOI] [PubMed] [Google Scholar]
- 79.Brown RS, Kumar KS, Russo MW, Kinkhabwala M, Rudow DL, Harren P. Model for end-stage liver disease and Child-Turcotte-Pugh score as predictors of pretransplantation disease severity, posttransplantation outcome, and resource utilization in United Network for Organ Sharing status 2A patients. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2002;8:278–284. doi: 10.1053/jlts.2002.31340. [DOI] [PubMed] [Google Scholar]
- 80.Linecker M, Krones T, Berg T, Niemann CU, Steadman RH, Dutkowski P. Potentially inappropriate liver transplantation in the era of the “sickest first” policy - A search for the upper limits. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 81.Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2008;8:2537–2546. doi: 10.1111/j.1600-6143.2008.02400.x. [DOI] [PubMed] [Google Scholar]
- 82.Rana A, Jie T, Porubsky M, Habib S, Rilo H, Kaplan B. The survival outcomes following liver transplantation (SOFT) score: validation with contemporaneous data and stratification of high-risk cohorts. Clin Transplant. 2013;27:627–632. doi: 10.1111/ctr.12181. [DOI] [PubMed] [Google Scholar]
- 83.Panchal HJ, Durinka JB, Patterson J, Karipineni F, Ashburn S, Siskind E. Survival outcomes in liver transplant recipients with Model for End-stage Liver Disease scores of 40 or higher: a decade-long experience. HPB. 2015;17:1074–1084. doi: 10.1111/hpb.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bosslet GT, Pope TM, Rubenfeld GD, Lo B, Truog RD, Rushton CH. An Official ATS/AACN/ACCP/ESICM/SCCM Policy Statement: Responding to Requests for Potentially Inappropriate Treatments in Intensive Care Units. Am J Respir Crit Care Med. 2015;191:1318–1330. doi: 10.1164/rccm.201505-0924ST. [DOI] [PubMed] [Google Scholar]
- 85.Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med. 2018;16 doi: 10.1186/s12916-018-1110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cardoso FS, Karvellas CJ, Kneteman NM, Meeberg G, Fidalgo P, Bagshaw SM. Postoperative resource utilization and survival among liver transplant recipients with Model for End-stage Liver Disease score ≥ 40: A retrospective cohort study. Can J Gastroenterol Hepatol. 2015;29:185–191. doi: 10.1155/2015/954656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levesque E, Winter A, Noorah Z, Daurès J-P, Landais P, Feray C. Impact of acute-on-chronic liver failure on 90-day mortality following a first liver transplantation. Liver Int Off J Int Assoc Study Liver. 2017;37:684–693. doi: 10.1111/liv.13355. [DOI] [PubMed] [Google Scholar]
- 88.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 89.Miskulin D, Bragg-Gresham J, Gillespie BW, Tentori F, Pisoni RL, Tighiouart H. Key comorbid conditions that are predictive of survival among hemodialysis patients. Clin J Am Soc Nephrol CJASN. 2009;4:1818–1826. doi: 10.2215/CJN.00640109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol Off J Am Soc Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 92.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 93.Charlson ME, Pompei P, Ales KL, Mac Kenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 94.Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2007;13:1515–1520. doi: 10.1002/lt.21172. [DOI] [PubMed] [Google Scholar]
- 95.VanWagner LB, Lapin B, Levitsky J, Wilkins JT, Abecassis MM, Skaro AI. High early cardiovascular mortality after liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2014;20:1306–1316. doi: 10.1002/lt.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.VanWagner LB, Ning H, Whitsett M, Levitsky J, Uttal S, Wilkins JT. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: The CAR-OLT score. Hepatol Baltim Md. 2017;66:1968–1979. doi: 10.1002/hep.29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Petrowsky H, Rana A, Kaldas FM, Sharma A, Hong JC, Agopian VG. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg. 2014;259:1186–1194. doi: 10.1097/SLA.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 98.Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708–715. doi: 10.1016/j.jhep.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 99.Schlegel A, Linecker M, Kron P, Györi G, De Oliveira ML, Müllhaupt B. Risk Assessment in High- and Low-MELD Liver Transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2017;17:1050–1063. doi: 10.1111/ajt.14065. [DOI] [PubMed] [Google Scholar]
- 100.Åberg F, Nordin A, Mäkisalo H, Isoniemi H. Who is too healthy and who is too sick for liver transplantation: external validation of prognostic scores and survival-benefit estimation. Scand J Gastroenterol. 2015;50:1144–1151. doi: 10.3109/00365521.2015.1028992. [DOI] [PubMed] [Google Scholar]
- 101.Lai JC. Defining the threshold for too sick for transplant. Curr Opin Organ Transplant. 2016;21:127–132. doi: 10.1097/MOT.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 104.Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatol Baltim Md. 2016;63:574–580. doi: 10.1002/hep.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2010;16:1373–1378. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 106.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatol Baltim Md. 2015;62:584–590. doi: 10.1002/hep.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tandon P, Reddy KR, O’Leary JG, Garcia-Tsao G, Abraldes JG, Wong F. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatol Baltim Md. 2017;65:217–224. doi: 10.1002/hep.28900. [DOI] [PubMed] [Google Scholar]
- 108.Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43:1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Le Maguet P, Roquilly A, Lasocki S, Asehnoune K, Carise E, Saint Martin M. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 110.Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 111.van Vugt JLA, Levolger S, de Bruin RWF, van Rosmalen J, Metselaar HJ, IJzermans JNM. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 112.Golse N, Bucur PO, Ciacio O, Pittau G, Sa Cunha A, Adam R. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2017;23:143–154. doi: 10.1002/lt.24671. [DOI] [PubMed] [Google Scholar]
- 113.Praktiknjo M., Book M., Luetkens J., Pohlmann A., Meyer C., Thomas D. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology. 2018;67:1014–1026. doi: 10.1002/hep.29602. [DOI] [PubMed] [Google Scholar]
- 114.Mueller N, Murthy S, Tainter CR, Lee J, Riddell K, Fintelmann FJ. Can Sarcopenia Quantified by Ultrasound of the Rectus Femoris Muscle Predict Adverse Outcome of Surgical Intensive Care Unit Patients as well as Frailty? A Prospective, Observational Cohort Study. Ann Surg. 2016;264:1116–1124. doi: 10.1097/SLA.0000000000001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huebener P, Sterneck MR, Bangert K, Drolz A, Lohse AW, Kluge S. Stabilisation of acute-on-chronic liver failure patients before liver transplantation predicts post-transplant survival. Aliment Pharmacol Ther. 2018;47:1502–1510. doi: 10.1111/apt.14627. [DOI] [PubMed] [Google Scholar]
- 116.Thuluvath PJ, Thuluvath AJ, Hanish S, Savva Y. Liver transplantation in patients with multiple organ failures: Feasibility and outcomes. J Hepatol. 2018 doi: 10.1016/j.jhep.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 117.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433–485. [Google Scholar]
- 118.Michard B, Artzner T, Lebas B, Besch C, Guillot M, Faitot F. Liver transplantation in critically ill patients: Preoperative predictive factors of post-transplant mortality to avoid futility. Clin Transplant. 2017 doi: 10.1111/ctr.13115. [DOI] [PubMed] [Google Scholar]
- 119.Karvellas CJ, Lescot T, Goldberg P, Sharpe MD, Ronco JJ, Renner EL. Liver transplantation in the critically ill: a multicenter Canadian retrospective cohort study. Crit Care Lond Engl. 2013;17:R28. doi: 10.1186/cc12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choudhury A, Kumar M, Sharma BC, Maiwall R, Pamecha V, Moreau R. Systemic inflammatory response syndrome in acute-on-chronic liver failure: Relevance of “golden window”: A prospective study. J Gastroenterol Hepatol. 2017;32:1989–1997. doi: 10.1111/jgh.13799. [DOI] [PubMed] [Google Scholar]
- 121.Lokhandwala S, McCague N, Chahin A, Escobar B, Feng M, Ghassemi MM. One-year mortality after recovery from critical illness: A retrospective cohort study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fröhlich S, Murphy N, Kong T, Ffrench-O’Carroll R, Conlon N, Ryan D. Alcoholic liver disease in the intensive care unit: outcomes and predictors of prognosis. J Crit Care. 2014;29:1131.e7–1131.e13. doi: 10.1016/j.jcrc.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 123.Warren A, Soulsby CR, Puxty A, Campbell J, Shaw M, Quasim T. Long-term outcome of patients with liver cirrhosis admitted to a general intensive care unit. Ann Intensive Care. 2017;7 doi: 10.1186/s13613-017-0257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levesque E, Saliba F, Ichaï P, Samuel D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014;60:570–578. doi: 10.1016/j.jhep.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 125.Heemann U, Treichel U, Loock J, Philipp T, Gerken G, Malago M. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatol Baltim Md. 2002;36:949–958. doi: 10.1053/jhep.2002.36130. [DOI] [PubMed] [Google Scholar]
- 126.Hassanein TI, Tofteng F, Brown RS, McGuire B, Lynch P, Mehta R. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatol Baltim Md. 2007;46:1853–1862. doi: 10.1002/hep.21930. [DOI] [PubMed] [Google Scholar]
- 127.Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatol Baltim Md. 2013;57:1153–1162. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 128.Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:782–789.e3. doi: 10.1053/j.gastro.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 129.Thompson J, Jones N, Al-Khafaji A, Malik S, Reich D, Munoz S. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2018;24:380–393. doi: 10.1002/lt.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Theocharis SE, Papadimitriou LJ, Retsou ZP, Margeli AP, Ninos SS, Papadimitriou JD. Granulocyte-colony stimulating factor administration ameliorates liver regeneration in animal model of fulminant hepatic failure and encephalopathy. Dig Dis Sci. 2003;48:1797–1803. doi: 10.1023/a:1025463532521. [DOI] [PubMed] [Google Scholar]
- 131.Spahr L, Lambert J-F, Rubbia-Brandt L, Chalandon Y, Frossard J-L, Giostra E. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatol Baltim Md. 2008;48:221–229. doi: 10.1002/hep.22317. [DOI] [PubMed] [Google Scholar]
- 132.Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505–512.e1. doi: 10.1053/j.gastro.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 133.Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109:1417–1423. doi: 10.1038/ajg.2014.154. [DOI] [PubMed] [Google Scholar]
- 134.Di Campli C, Zocco MA, Saulnier N, Grieco A, Rapaccini G, Addolorato G. Safety and efficacy profile of G-CSF therapy in patients with acute on chronic liver failure. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2007;39:1071–1076. doi: 10.1016/j.dld.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 135.Simonetto DA, Shah VH, Kamath PS. Improving survival in ACLF: growing evidence for use of G-CSF. Hepatol Int. 2017;11:473–475. doi: 10.1007/s12072-017-9834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McPhail MJW, Auzinger G, Bernal W, Wendon JA. Decisions on futility in patients with cirrhosis and organ failure. Hepatol Baltim Md. 2016;64:986. doi: 10.1002/hep.28539. [DOI] [PubMed] [Google Scholar]
- 137.Shrime MG, Ferket BS, Scott DJ, Lee J, Barragan-Bradford D, Pollard T. Time-Limited Trials of Intensive Care for Critically Ill Patients With Cancer: How Long Is Long Enough? JAMA Oncol. 2016;2:76–83. doi: 10.1001/jamaoncol.2015.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meropol NJ, Egleston BL, Buzaglo JS, Benson AB, Cegala DJ, Diefenbach MA. Cancer patient preferences for quality and length of life. Cancer. 2008;113:3459–3466. doi: 10.1002/cncr.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Danis M, Patrick DL, Southerland LI, Green ML. Patients’ and families’ preferences for medical intensive care. JAMA. 1988;260:797–802. [PubMed] [Google Scholar]