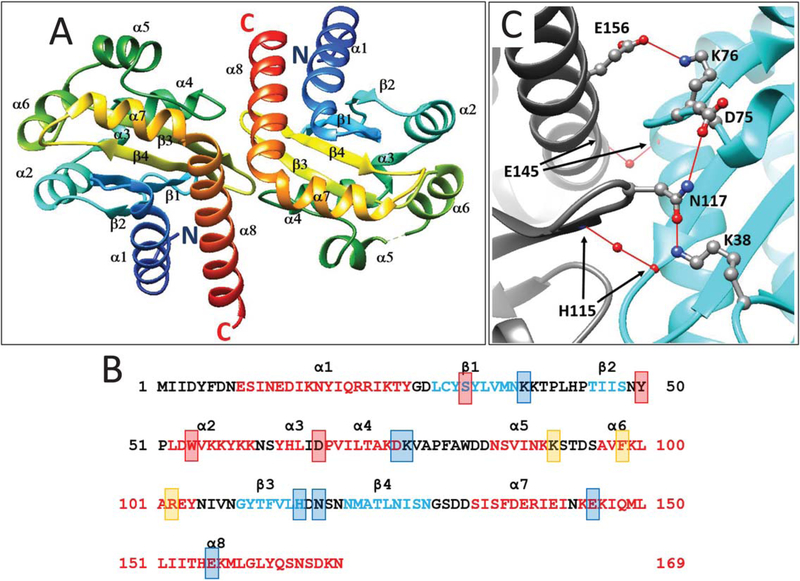
FIGURE 1.
A. Ribbon diagram of a dimer of the YenR-LBD in the absence of pheromone. Colors indicate position from the N terminus (blue) to C terminus (red). α helices and β strands are indicated and numbered from N terminus to C terminus. B. Amino acid sequence of YenR-LBD. Helices α1-α8 are depicted using red letters while strands β1-β4 are depicted using blue letters. Amino acid residues that make hydrogen bonds with OHHL are depicted using red shaded boxes, while those important for subunit interactions are shown using blue shaded boxes, and those at the opening of the OHHL binding cavity are shown using orange boxes. C. Subunit interface of the YenR-LBD dimer. A salt bridge is formed between a side-chain oxygen of Glu156 of each subunit and the side-chain amine of Lys76 of the opposite subunit. For clarity, only one such bond is shown. A hydrogen bond is formed between the side-chain oxygen of Asn117 of one subunit and the side-chain amine of Lys38 of the opposite subunit, while another hydrogen bond is formed between the side-chain amine of Asn117 and a side-chain oxygen of Asp75. A water-mediated hydrogen bond is formed between the backbone nitrogen of His115 of each subunit and the backbone oxygen of His115 of the opposite subunit. A second water-mediated hydrogen bond is formed between the backbone oxygen of Glu145 of each subunit [Color figure can be viewed at wileyonlinelibrary.com]