FIGURE 3.
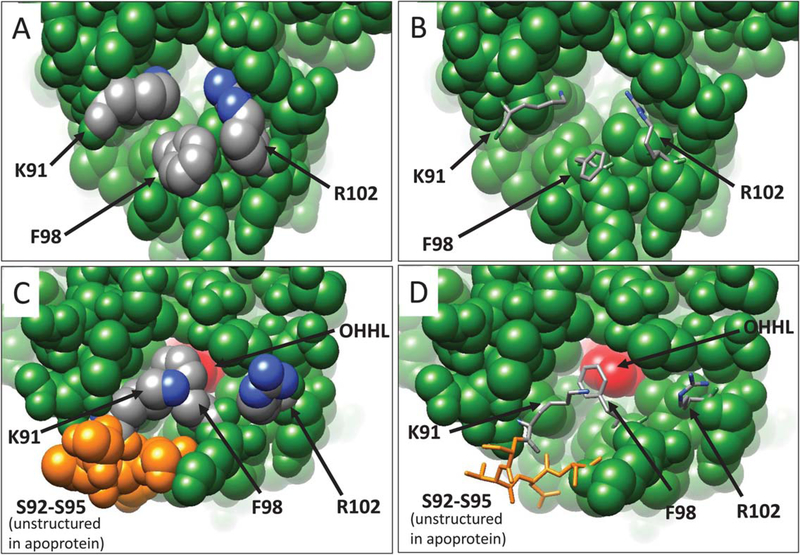
YenR apoprotein has an aqueous channel that maintains access to the OHHL binding site. The side chains of Lys91 and Arg102 are oriented inward and may be flexible in solution (Figure 3A,B). In contrast, Phe98 is oriented outward to solvent in the apoprotein. Ser92-Ser95 are disordered and not visible in the apoprotein. In the presence of OHHL, Phe98 swings inward and makes hydrophobic contacts with the pheromone, while Lys91 and Arg102 swing outward to the solvent (Figure 3C,D). Ser92-Ser95 are structured in the presence of OHHL (Figure 3C,D, residues in orange). These changes effectively seal the pheromone within the protein. In parts A and C, all residues are depicted as space-filling spheres, while in parts B and D, critical residues are depicted as stick figures. OHHL is shown in red