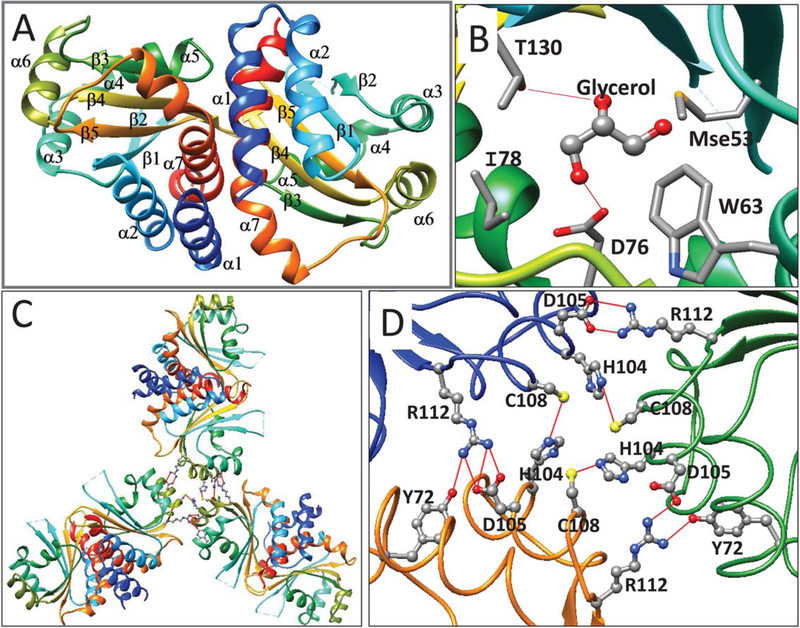
FIGURE 5.
(A) Ribbon diagram of apo-CepR2-LBD. Coloring and labelling are as described in Figure 1. (B) CepR2-LBD is complexed with one molecule of glycerol in the presumptive OHL binding site. Glycerol forms hydrogen bonds to Thr130 and Asp76 and makes van der Waals contacts to Ile78, Trp63 and Mse53. Trp63, Asp76, and Thr130 correspond to residues of homologous proteins that make hydrogen bonds with AHLs (see Supporting Information Figure S2). (C) The CepR-LBD asymmetric unit contains three dimers and shows three-fold rotational symmetry. D. Trimers of dimers of CepR2-LBD are stabilized by interprotein hydrogen bonds between Cys108 and His104 and between Arg112 and Asp105. In two subunits, Tyr72 also bonds to Arg112 [Color figure can be viewed at wileyonlinelibrary.com]