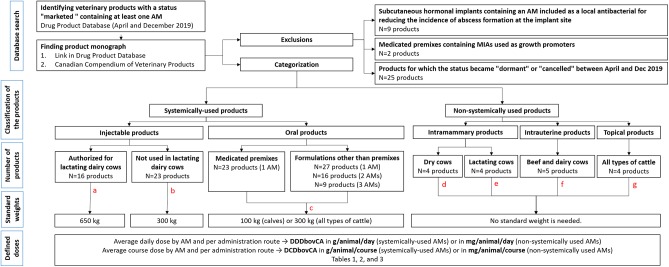
Figure 1.
Flow chart illustrating workflow for proposing DDDbovCA and DCDbovCA values for antimicrobial agents used for cattle in Canada. AM(s), Antimicrobial Agent(s); DDDbovCA, Canadian Defined Daily Dose for cattle; DCDbovCA, Canadian Defined Course Dose for cattle; MIA(s), Medically Important Antimicrobial(s) belonging to the Categories I, II, and III according to Health Canada. aInjectable products authorized for lactating dairy cows were assigned to a standard weight of 650 kg. bInjectable products not used in lactating dairy cows were assigned to a standard weight of 300 kg. cOral products intended for “calves” or “calves up to 136 kg” were assigned to a standard weight of 100 kg. Oral products intended for all types of cattle or for “calves up to 360 kg” were assigned to a standard weight of 300 kg. dFor intramammary products for dry cows, a complete treatment for a cow was defined as the infusion of four syringes (one per quarter) at drying off regardless of the product. The complete treatment (4 syringes) was assigned to a duration of action of 10 days meaning that the daily treatment was defined as the infusion of 0.4 syringe per cow, or 0.1 syringe per quarter. eFor intramammary products for lactating cows, it was hypothesized that one quarter is infected (and then treated) at a time by cow. fFor intrauterine products, when no indication could be found on the product monograph, a duration of action of 24 h was assigned by default. gFor topical products, we assumed that 1 mL is sprayed per second (for sprays), 5 g of cream is used per application (for creams), or 5 g of powder is used per application (for powders for topical administration).