Figure 5.
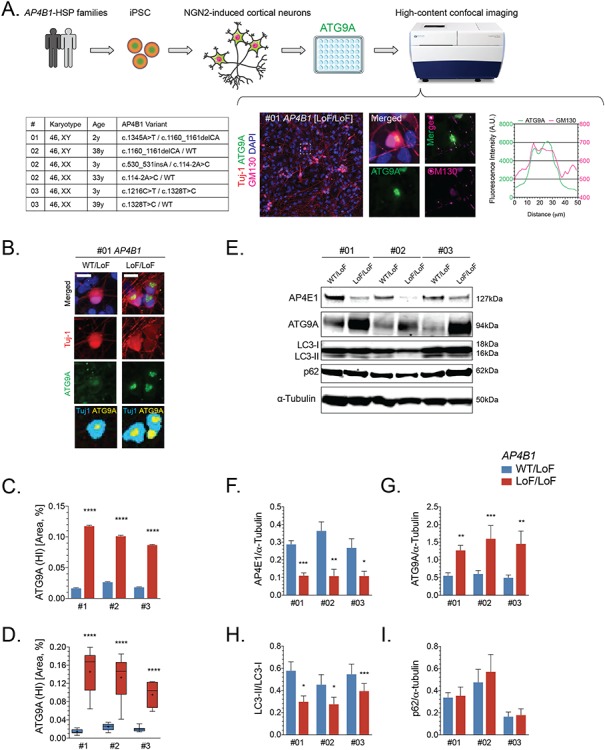
ATG9A is mislocalized in iPSC-derived cortical neurons from AP-4-HSP patients. (A) Fibroblasts from three families with AP4B1-associated HSP were reprogrammed into iPSCs and subsequently into excitatory cortical neurons using overexpression of neurogenin 2. iPSC-derived neurons were grown in 96 well plates and subjected to high-content confocal imaging to assess the localization of ATG9A. Immunocytochemistry was performed for the neuronal marker Tuj-1 (red), ATG9A (green), Golgi marker GM130 (pink) and nuclear marker DAPI (blue). ATG9A concentrated to a high-intensity juxtanuclear area that overlapped with GM130 with lower fluorescence intensity in the remainder of the soma. (B) In neurons from heterozygous controls, ATG9A was distributed in the soma, and the juxtanuclear high-intensity area (marked in yellow), if present, was often small. A significant increase in the high intensity juxtanuclear ATG9A area occurred in patient-derived neurons. The ratio between soma size by Tuj-1 staining (marked in cyan) and the ATG9A high intensity area (marked yellow) was significantly increased in cortical neurons from AP-4-HSP patients compared to controls. This was quantified on (C) a per cell basis with over 90x103 neurons analyzed per condition or (D) per differentiation with three independent rounds of differentiation per pair of cell lines. (E–I) Western blotting of whole cell lysates of iPSC-derived neurons from three patients with bi-allelic variants in AP4B1 reveals a reduction in the levels of AP4E1, a subunit and surrogate for levels of the AP-4 complex, increased levels of ATG9A and a decrease in the ratio of LC3-II to LC3-I. Scale bar: 20 μm. HI, high intensity; iPSC, induced pluripotent stem cells; LoF, loss of function; NGN2, neurogenin 2; WT, wild type.
