Abstract
The lack of cerebral creatine (Cr) causes intellectual disability and epilepsy. In addition, a significant portion of individuals with Cr transporter (Crt) deficiency (CTD), the leading cause of cerebral Cr deficiency syndromes (CCDS), are diagnosed with attention-deficit hyperactivity disorder. While the neurological effects of CTD are clear, the mechanisms that underlie these deficits are unknown. Part of this is due to the heterogenous nature of the brain and the unique metabolic demands of specific neuronal systems. Of particular interest related to Cr physiology are dopaminergic neurons, as many CCDS patients have ADHD and Cr has been implicated in dopamine-associated neurodegenerative disorders, such as Parkinson’s and Huntington’s diseases. The purpose of this study was to examine the effect of a loss of the Slc6a8 (Crt) gene in dopamine transporter (Slc6a3; DAT) expressing cells on locomotor activity and motor function as the mice age. Floxed Slc6a8 (Slc6a8flox) mice were mated to DATIREScre expressing mice to generate DAT-specific Slc6a8 knockouts (dCrt−/y). Locomotor activity, spontaneous activity, and performance in the challenging beam test were evaluated monthly in dCrt−/y and control (Slc6a8flox) mice from 3 to 12 months of age. dCrt−/y mice were hyperactive compared with controls throughout testing. In addition, dCrt−/y mice showed increased rearing and hindlimb steps in the spontaneous activity test. Latency to cross the narrow bridge was increased in dCrt−/y mice while foot slips were unchanged. Taken together, these data suggest that the lack of Cr in dopaminergic neurons causes hyperactivity while sparing motor function.
Keywords: creatine, creatine transporter, DAT-Cre, hyperactivity, motor function
Introduction
Creatine (Cr) is a nitrogenous acid involved in energy homeostasis. Through a Cr kinase (CK)-mediated process, Cr is phosphorylated at sites of energy production and diffuses through the cytosol, creating phospho-Cr (PCr) pools (Wallimann, Tokarska-Schlattner et al. 2011, Lowe, Kim et al. 2013). At sites of ATP consumption CK transfers the phosphate from PCr to ADP (Wyss and Kaddurah-Daouk 2000). The Cr/PCr shuttle is a spatial and temporal ATP buffer, preventing transient changes in ATP:ADP or ATP:AMP ratios from increasing mitochondrial production (Wallimann, Tokarska-Schlattner et al. 2011). Reflecting the importance of Cr in energetically demanding tissue, the highest Cr concentrations are seen in the brain, heart, and muscle. In addition, Cr has antioxidant, neuroprotective, and mitochondria-protective capabilities (Wyss and Kaddurah-Daouk 2000). The significance of brain Cr is highlighted by the hallmark phenotype of intellectual disability and aphasia in cerebral Cr deficiency syndromes (CCDS) (Cecil, Salomons et al. 2001, deGrauw, Salomons et al. 2002, van de Kamp, Betsalel et al. 2013). Cr transporter (Crt; SLC6A8) deficiency (CTD) is the most prevalent type of CCDS and is thought to be responsible for ~2% of all X-linked intellectual disability (Rosenberg, Almeida et al. 2004, Newmeyer, Cecil et al. 2005). While intellectual disability is the most striking feature of CTD, the incidence of motoric impairments are high among CTD patients (van de Kamp, Betsalel et al. 2013, van de Kamp, Mancini et al. 2014). To date, it is unclear how the loss of Cr leads to cognitive deficits. One of the primary challenges in elucidating the mechanisms of Cr-mediated deficits is that the importance of Cr in specific neurotransmitter systems is unknown. This limits the ability to focus on a distinct cellular process that might be exclusive to one neuronal cell type and could be overlooked by global evaluation of the brain.
Dopamine (DA) is the primary neurotransmitter involved in voluntary motor function (Beninger 1983). Many movement disorders, e.g. Parkinson’s and Huntington’s disease, are caused by disruptions in dopaminergic function. Drugs that increase DA release, such as amphetamine and cocaine, cause hyperactivity (Roberts, Zis et al. 1975, Faraone 2018, Runegaard, Sorensen et al. 2018). Conversely, drugs that deplete DA result in hypoactivity and motor deficits (Koob, Riley et al. 1978, Beninger 1983). Additionally, DA neurons are especially sensitive to mitochondrial deficits. 6-hydroxydopamine (6-OHDA) (Gomez-Lazaro, Galindo et al. 2008), rotenone (Xiong, Long et al. 2012), and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Przedborski, Tieu et al. 2004) are mitochondrial toxicants that degenerate DA neurons and cause motor dysfunction. As such, they are regularly used to model Parkinson’s disease (Sakai and Gash 1994, Matthews, Ferrante et al. 1999, Przedborski, Tieu et al. 2004, Tirmenstein, Hu et al. 2005, Hosamani, Ramesh et al. 2010, Glajch, Fleming et al. 2012, Xiong, Long et al. 2012). Importantly, Cr supplementation prevents or attenuates MPTP- (Matthews, Ferrante et al. 1999, Klivenyi, Calingasan et al. 2004), 6-OHDA- (Cunha, Martin-de-Saavedra et al. 2013, Cunha, Martin-de-Saavedra et al. 2014), and rotenone-induced toxicity (Hosamani, Ramesh et al. 2010) suggesting that Cr could be an important mediator of mitochondrial-induced motor impairments.
To better understand the role of brain Cr, we developed transgenic mice with exons 2-4 of the murine Slc6a8 (Crt) gene flanked by loxP sites (Slc6a8Flox; Skelton et al., 2011). The Slc6a8 gene is responsible for the transport of Cr into cells (Wyss and Kaddurah-Daouk 2000). Ubiquitous knockout (Slc6a8−/y) mice generated from this line have reduced cerebral Cr, lower body weight, show significant learning and memory deficits (Skelton, Schaefer et al. 2011, Hautman, Kokenge et al. 2014, Ullio-Gamboa, Udobi et al. 2019), and importantly, have increased striatal DA concentrations (Skelton, Schaefer et al. 2011, Abdulla, Pennington et al. 2019). Additionally, Slc6a8−/y mice have reduced swim speed compared with control (Slc6a8+/y) mice (Skelton, Schaefer et al. 2011), indicative of the potential presence of motor impairments. Interestingly, Slc6a8−/y mice were hypoactive during the first five minutes of a one hour open field test, however, female Slc6a8+/− (Hautman, Kokenge et al. 2014) and male brain-specific Slc6a8 knockout mice (Udobi, Kokenge et al. 2018) were hyperactive for the whole hour. This would suggest that Cr plays a role in exploratory behavior or activity levels. In order to isolate Slc6a8’s influence on the dopaminergic system, and to reduce potential confounds created by the size differences in ubiquitous knockout mice, we created a DA-specific Slc6a8 knockout mouse (dCrt−/y). The purpose of this study was to determine the effect of a DA-specific Slc6a8 knockout on locomotor activity and motor performance during adulthood.
Experimental Procedures
Subjects
Slc6a3tm1.1(cre)Bkmn/J (DATIREScre) mice were obtained from Jackson Labs (Bar Harbor, ME) (Backman, Malik et al. 2006). DATIREScre+ mice were bred to wild-type mice from the same background strain (C57Bl/6J) for at least one generation to establish an in-house colony. In-house generated female Slc6a8fl/fl mice (Skelton et al., 2011) were mated with the in-house generated male DATIREScre+ mice. The resulting offspring were used as subjects for these studies with no more than 1 mouse/genotype/litter represented. Male Slc6a8fl/y:: DATIREScre+ (dCrt−/y) and Slc6a8fl/y:: DATIREScre− (Slc6a8fl/y) were tested every 30 days beginning at postnatal day (P) 90 and ending on P360. Hyperactivity has not been observed in either the Slc6a8fl/y (Udobi, Kokenge et al. 2018, Udobi, Delcimmuto et al. 2019) or the heterozygous DATIREScre mice (Birgner, Nordenankar et al. 2010) when compared with wild-type mice, so we selected the Slc6a8fl/y littermates as controls. Mice were weighed monthly prior to running behavioral assessments. Thirteen Slc6a8fl/y and 11 dCrt−/y mice were enrolled at the beginning of the study and the study concluded with 8 Slc6a8fl/y and 7 dCrt−/y mice. There was no clear cause of death for the mice that were lost during testing and none of the deaths were related to testing. A separate group of 3 dCrt−/y, 3 Slc6a8+/y, and 3 Slc6a8−/y mice were used for in situ hybridization. All procedures were performed in the Cincinnati Children’s Research Foundation vivarium which is fully accredited by AAALAC International and protocols were approved by the Institutional Animal Use and Care Committee (protocol #2017-0019). Mice (2-4/cage) were housed in microisolators equipped with an automatic watering system. Lights were on a 14:10 ligh:dark cycle, room temperature was maintained at 19±1° C. Sterilized food and water were available ad libitum. All institutional and national guidelines for the care and use of laboratory animals were followed.
Behavior
Challenging Beam Task.
The protocol was adapted from Fleming et al. (2004) with minor changes. A 1 m beam was divided into four-25 cm segments: the first was 3.5 cm wide and the width of each subsequent segment was reduced in 1 cm increments to a final width of 0.5 cm. A wire mesh grid (0.5 cm × 0.5 cm) was placed atop the beam. The bridge was elevated 13 cm from the tabletop using standard barrier mouse cages. Mice were trained for two days prior to testing. On day one, mice were assisted as they traversed the 1 m beam to the home-cage goal for two trials. Day one concluded when mice were able to complete 5 unassisted bridge traversals. On the second day of training, mice were required to complete 5 unassisted bridge traversals. For testing, mice were assessed over three, 2 min trials. Trials were recorded and scored by a trained observer blinded to genotype. The variables measured were latency to cross (2 min maximum), errors – defined as a foot-slip during forward progress – committed per steps taken, and total number of steps (Fleming et al., 2004).
Spontaneous Activity.
A transparent glass cylinder (15.5 cm [h] × 12.7 cm [dia]) was placed on a plexiglass platform with a camera below to record activity. Mice were placed in the cylinder for 3 min. Trials were recorded and scored offline by a trained observer blinded to genotype. The dependent measures were steps per limb (front and hind limb), rears, and total time grooming.
Overnight Locomotor Activity.
Locomotor activity was assessed in automated activity monitoring chambers (40 cm [w] × 40 cm [d] × 38 cm [h]) with 16 LED-photodetector beams in the X and Y planes (Photobeam Activity System – Open Field, San Diego Instruments, San Diego, CA). Photocells were spaced 2.5 cm apart. Mice were tested for 14 h, consisting of the last 2 h of the light phase, the 10 h dark phase and 2 h of the following light phase.
In Situ Hybridization
Mice were anesthetized with isoflurane, rapidly decapitated, and the brains dissected. Fresh-frozen sections through the ventral midbrain were cut on a cryostat (at 10 μm thickness) and slide-mounted onto Superfrost plus microslides (VWR, Batavia, IL). Semi-adjacent sections were processed for the in situ hybridization localization of Slc6a8 and tyrosine hydroxylase (TH) mRNAs using 35S-labeled riboprobes, as described in detail previously (Seroogy and Herman 1997, Numan, Gall et al. 2005, Hemmerle, Dickerson et al. 2012). Briefly, for pretreatment, the sections were placed in 4% paraformaldehyde (10 min), rinsed in 0.1M phosphate-buffered saline (PBS) (5 min; twice) and 0.1M PBS/0.2% glycine (5 min; twice), acetylated in triethanolamine (pH 8.0) containing 0.25% acetic anhydride (10 min), dehydrated in a series of ethanol washes, delipidated in chloroform and air-dried. Sections were hybridized overnight in a humidified chamber at 60° C in hybridization solution with the 35S-labeled probe at a concentration of 3×105 cpm/50 μl. The composition of the hybridization cocktail for each probe was as follows: 50% deionized formamide, 10% dextran sulfate, 20 mM Tris-HCl, 10 mM EDTA, 40 mM dithiothreitol, 1X Denhardt’s, 335 mM NaCl, 0.3 mg/ml denatured salmon sperm DNA, 0.15 mg/ml tRNA, DEPC H2O, and the appropriate 35S-labeled cRNA probe. The TH cDNA plasmid (kindly provided by J. Herman, University of Cincinnati) was contained in a pCR-TOPO vector and consisted of 366 bp. The templates for the 515 bp Slc6a8 sense and antisense probes were generated by PCR amplification of exons 2-4 of murine Slc6a8 mRNA. Total RNA was extracted from the hippocampus of a C57BL/6J mouse using Trizol (Invitrogen, Carlsbad, CA) and a cDNA library was generated using iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA). For the antisense probe, a T3 promoter was added to the 5’ prime end of the reverse primer (5’ AATTAACCCTCACTAAAGGTGTGTTCCTTATTCCCTA 3’, T3 sequence underlined) and the sense probe was generate by adding the T7 promoter to the 5’ end of the forward primer (5’ TAATACGACTCACTATAGGGCTTTCCTGTTGACTTGACC 3’; T7 sequence underlined). Probes were generated by in vitro transcription and labeled by incorporation of 35S-ATP. Following hybridization, coverslips were removed and the sections were taken through a series of post-hybridization washes in decreasing concentrations of standard sodium citrate, treated with ribonuclease A, rinsed again, and air-dried. The sections were then exposed to BioMax MR film (Kodak, Rochester, NY) for 2 days (TH cRNA) or 14 days (Crt cRNA) for generation of film autoradiograms. The films were developed with Kodak GBX developer and fixer (Kodak). Images were scanned at 3200 dpi from the film autoradiograms using Epson Scan software (Epson America, Inc., Long Beach, CA).
Statistics
Repeated-measures two-way ANOVAs (age × genotype) were used for all analyses in this study. Significant interactions were evaluated using post hoc analysis with the two-stage step up procedure of Benjamini, Krieger, and Yekutieli (Benjamini, Krieger et al. 2006). Significance was set at p<0.05 for all tests. Data were analyzed and graphs were generated using GraphPad Prism version 8.2.0 for Windows (GraphPad Software, La Jolla, CA).
Results
Weight.
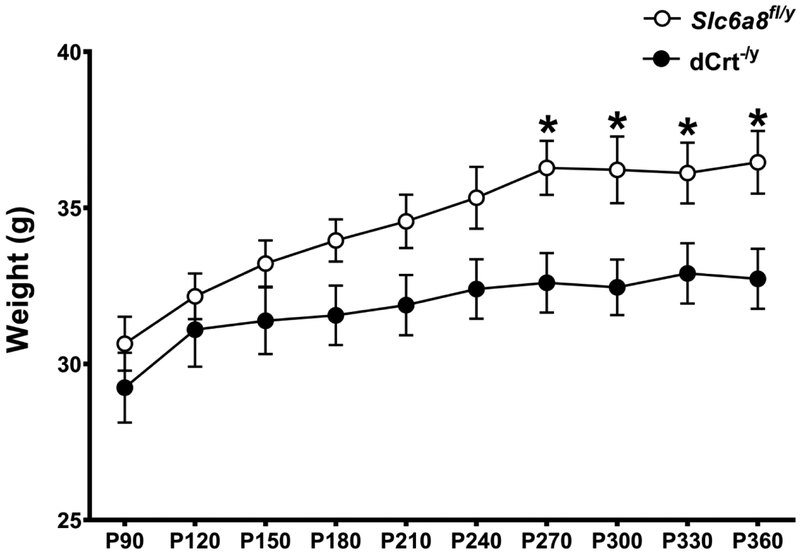
While there were no weight differences between groups when the mice were younger, dCrt−/y mice did show a weight reduction from P270 to P330 compared with Slc6a8−/y mice (age × gene interaction: F(9,153)=1.95, p<0.05; Fig. 1). All mice gained weight as they aged (F(9,153)=18.92, p<0.0001).
Fig. 1. Weight.
An age × gene interaction revealed that dCrt−/y mice weighed less than Slc6a8fl/y mice from P270 to P330. All animals gained weight as they aged. (*=p<0.05)
Challenging Beam.
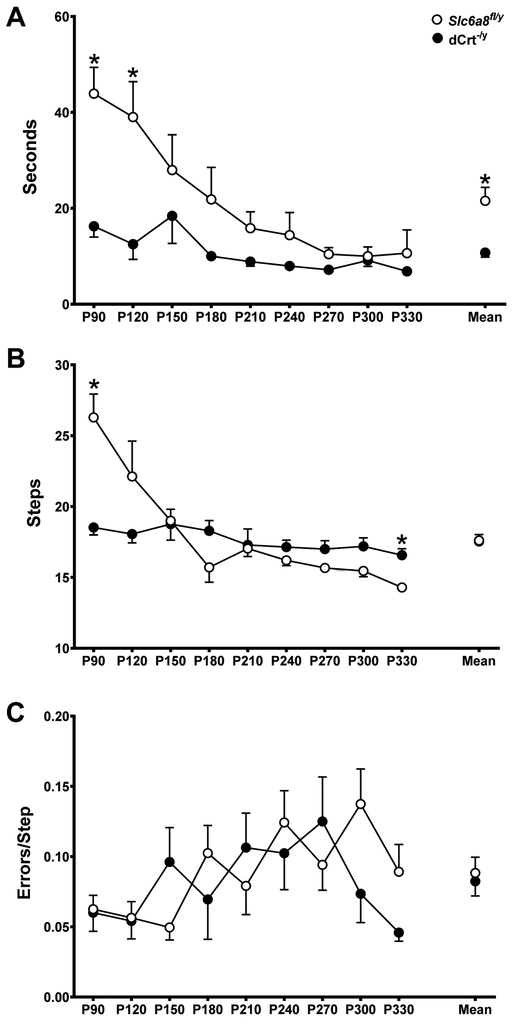
dCrt−/y mice traversed the beam faster than Slc6a8fl/y mice and the latency to cross decreased as mice aged (main effect of gene: (F(1,152)=19.98, p<0.0001); main effect of age: F(8,152)=5.03, p<0.001; Fig. 2A). On P90 dCrt−/y mice took fewer steps to cross the beam than Slc6a8fl/y mice but took more steps on P330 (age × gene interaction: F(9,116)=5.77, p<0.001; Fig. 2B). For number of errors committed there were no differences between groups (Fig. 2C).
Fig. 2. dCrt−/y mice have decreased beam crossing latencies.
(A) dCrt−/y mice traversed the beam faster than Slc6a8fl/y animals and the latency to cross decreased as mice aged. (B) An age × gene interaction was reported for number of steps required to cross the beam, with dCrt−/y mice requiring less steps on P90 and more steps on P330. (C) Groups did not differ on number of errors committed.
Spontaneous Activity.
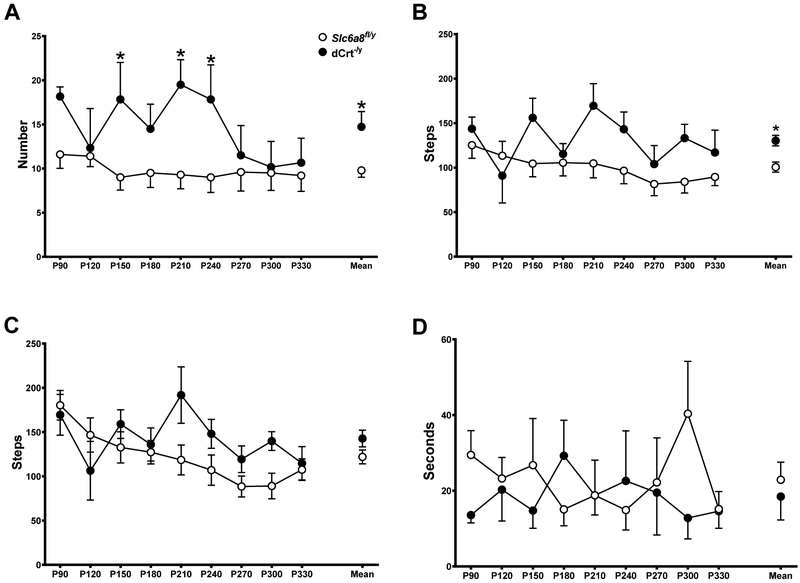
dCrt−/y mice reared more (main effect of gene: F(1,14)=8.899, p<0.01; Fig. 3A) and had more hindlimb steps (main effect of gene: F(1,14)=11.8, p<0.01; Fig. 3B) than Slc6a8fl/y mice. Regardless of genotype, mice had fewer forelimb steps as they aged (main effect of age: F(8,112)=3.465, p<0.05; Fig. 3C). No differences were found for grooming duration (Fig. 3D).
Fig. 3. dCrt−y mice have increased spontaneous activity.
(A, B) dCrt−/y mice reared more and (C, D) took more hindlimb steps than Slc6a8fl/y mice, but recorded similar activity on (E,F) and forelimb steps (G, H) and grooming duration
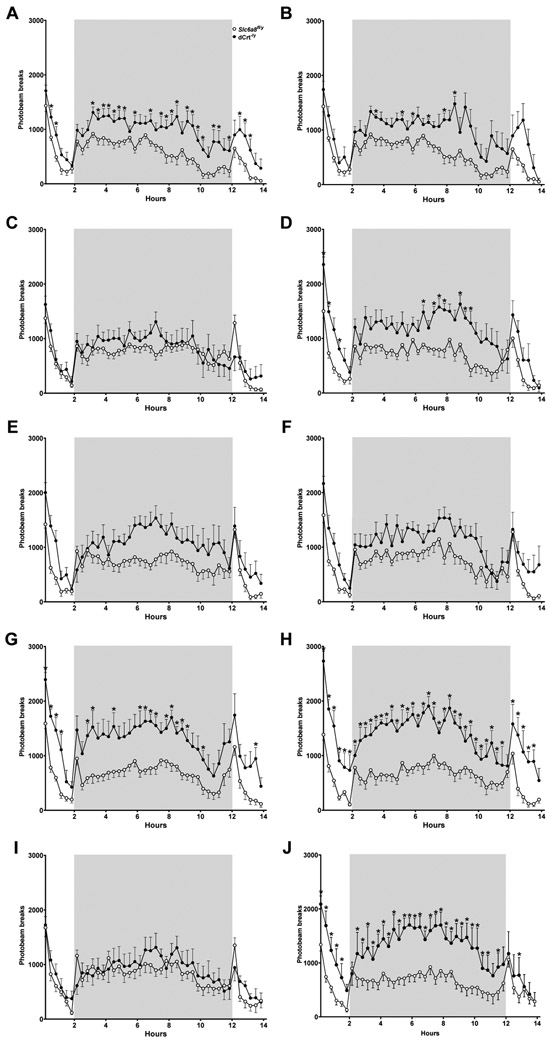
Overnight Locomotor Activity.
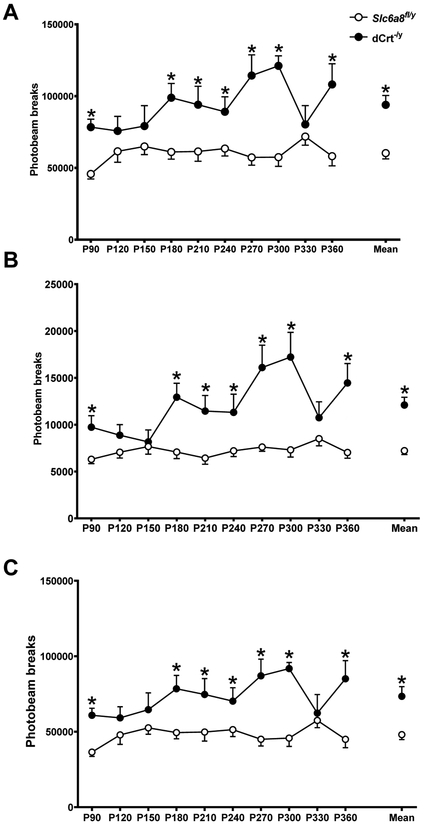
dCrt−/y mice were hyperactive compared with Slc6a8fl/y mice at all ages except P120, P150, and P330 (main effect of gene: F(1,16)=21.09, p<0.001; main effect of age: F(9,144)=3.89, p<0.001; age × gene interaction: F(9,144)=3.88, p<0.001; Fig. 4A, Fig. 5). Hyperactivity was present during both the light phase (main effect of gene: F(1,16)=35.51, p<0.0001; main effect of age: F(9,144)=5.05, p<0.0001; age × gene interaction: F(9,144)=4.78, p<0.0001; Fig. 4B, Fig. 5) and the dark phase (main effect of gene: F(1,16)=15.42, p<0.01; main effect of age: F(9,144)=2.56, p<0.01; age × gene interaction: F(9,144)=3.49, P<0.001; Fig. 4C, Fig. 5) for all ages except P120, P150, and P330.
Fig. 4. Persistent hyperactivity in dCrt−/y mice.
(A) dCrt−/y mice had increased activity compared with Slc6a8fl/y mice at all ages except P120, P150, and P330. During both the (B) light and (C) dark phases of the experiment, dCrt−/y mice were more active than Slc6a8fl/y controls on all testing periods except P120, P150, and P330
Fig. 5. Overnight locomotor activity.
dCrt−/y mice are hyperactive compared to Slc6a8fl/y mice throughout testing. 14-hour locomotor assessments from (A) P90, (B) P120, (C) P150, (D) P180, (E) P210, (F) P240, (G) P270, (H) P300, (I) P330, and (J) P360 with the dark phase contained within the gray box.
In Situ Hybridization.
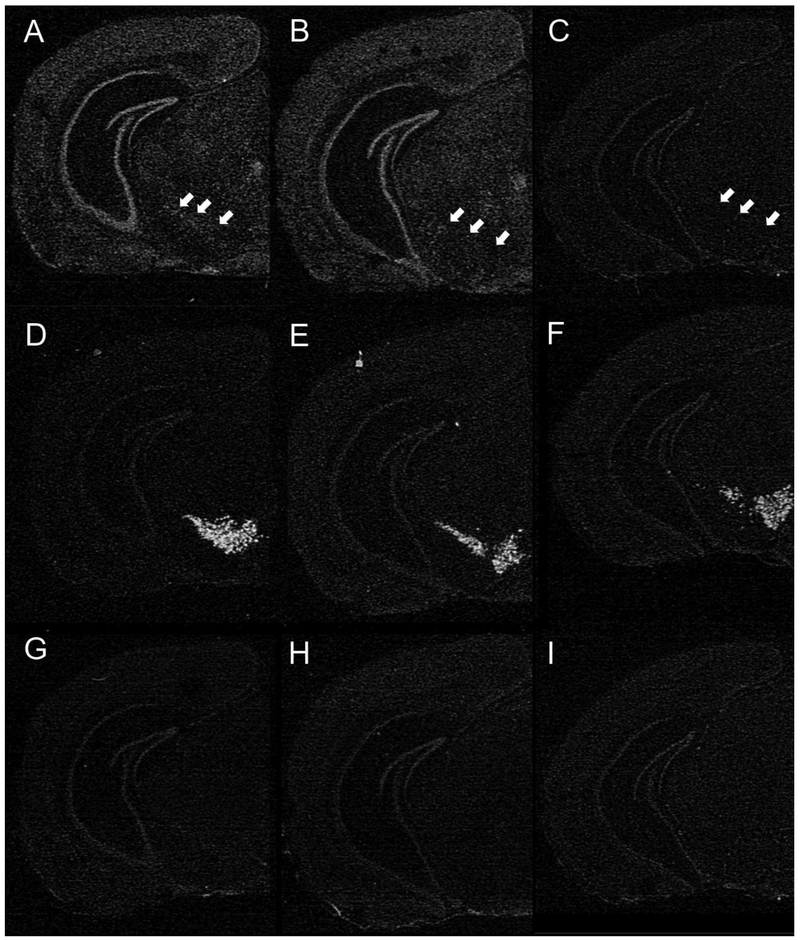
Widespread expression of Slc6a8 mRNA was observed in brain sections of Slc6a8+/y and dCrt−/y mice, but, as expected, expression was not present in Slc6a8−/y mouse brains (Fig. 6A-C). Representative autoradiograms from the dCrt−/y mice demonstrated that Slc6a8 cRNA hybridization was absent specifically in the region of the ventral midbrain corresponding to ventral midbrain dopaminergic nuclei, as determined by processing semi-adjacent sections for TH mRNA localization (Fig. 6D-F). No labeling was present using the sense Slc6a8 riboprobe (Fig. 6G-I). These results provide strong evidence that the knockout of Slc6a8 mRNA was restricted to DAT-containing midbrain dopaminergic neurons in the dCrt−/y mice.
Fig. 6. Confirmation of knockout.
(A-C) Film autoradiograms showing hemisections at the level of the ventral midbrain hybridized with either antisense Slc6a8 cRNA, (D-F) TH cRNA or (G-I) control sense Slc6a8 cRNA. Robust Slc6a8 cRNA-labeling is present throughout the sections from the (A) Slc6a8+/y and (B) dCrt−/y mice; note, however, the absence of hybridization signal in the substantia nigra in the dCrt−/y brain section (arrows in B; compare to same area in A). (C) Labeling for Slc6a8 mRNA is absent (only typical background expression) in sections from Slc6a8−/y mouse brains. Panels D-F show TH cRNA labeling in sections semi-adjacent to panels A-C, respectively, and serve to identify the corresponding localization of dopaminergic cells in the ventral midbrain. Hybridization of semi-adjacent sections from each mouse genotype with the control sense Slc6a8 cRNA probe reveals complete absence of labeling for Slc6a8 mRNA (G-I)
Discussion
dCrt−/y mice traversed the challenging beam faster than controls, exhibited more stepping activity in spontaneous activity, and were hyperactive in overnight locomotor assessments. These results were consistent, not progressive, across the duration of this experiment, indicating that deletion of Slc6a8 in dopaminergic neurons results in persistent hyperactivity in mice. Additionally, in situ hybridization confirmed the successful targeted deletion of Slc6a8 in DA neurons, as minimal expression of Slc6a8 mRNA was observed in TH-rich regions of the dCrt−/y brain. Our results suggest that the Slc6a8 gene plays an important role in DA-mediated locomotion.
The challenging beam task was designed to uncover subtle motor deficits that could provide translational value to human neurodegenerative disorders (Fleming et al., 2004). For instance, mice exhibiting a Parkinson’s-like phenotype display increased errors, steps to cross, and latency to cross the beam (Fleming et al., 2004; Glajch et al., 2012). dCrt−/y mice did not show any deficits on challenging beam task. Instead, dCrt−/y mice had shorter latencies when crossing the tapered beam and never relied on more steps to cross the beam than control mice. Coupled with a lack of errors on this task, dCrt−/y performance on the challenging beam task appear to be mediated by a hyperactivity that apparently does not impair motor control.
Furthermore, in the spontaneous activity task, dCrt−/y mice had increased rearing behavior and hindlimb steps compared with Slc6a8fl/y mice. Mice with motor deficits typically display a decrease in these behaviors (Schallert et al., 2000; Fleming et al., 2004; Glajch et al., 2012; Pinto et al., 2016). These increases are indicative of hyperactivity (O’Neill and Gu, 2013; Kostrzewa et al., 2015). Similarly, dCrt−/y mice were hyperactive in overnight locomotor activity assessments during both light and dark phases, compared with Slc6a8fl/y controls. This hyperactivity was observed in 70% of the assessments. Taken together these behavioral measures indicate that the dCrt−/y mice are hyperactive.
The purpose of this study was to evaluate the effect of DAT-specific deletion of Slc6a8 affected motor behavior or activity levels. The results suggest that deletion of the Slc6a8 gene in DA neurons is sufficient to induce a persistent, life-long hyperactivity while sparing motor function. CTD patients are frequently diagnosed with attention-deficit/hyperactivity disorder (ADHD) (van de Kamp et al., 2013). There are logistical issues, such as the size, distribution, and heterogeneity of brain regions containing DAT+ neurons, that prevent Cr measurement in dopaminergic areas of dCrt−/y mice. However, multiple lines of evidence would suggest that deletion of Slc6a8 leads to a significant reduction in Cr levels. Slc6a8 appears to be the only means of Cr entry into neurons (Ohtsuki, Tachikawa et al. 2002). This is shown by the lack of brain Cr in brain-specific Slc6a8 knockouts (Udobi, Kokenge et al. 2018), where Slc6a8 expression is likely unchanged in the microcapillary endothelial cells that allow Cr entry into the brain, and the decline of brain Cr following Slc6a8 deletion in adult mice (Udobi, Delcimmuto et al. 2019). Further, the expression of Cr synthesis enzymes AGAT and GAMT are relatively low in the substantia nigra (Braissant, Henry et al. 2001). Therefore, it is possible but not likely that the Slc6a8 deletion did not alter Cr levels in these regions. It should be noted that despite the expression of AGAT and GAMT there has been no conclusive proof that the brain generates a significant amount of Cr through de novo synthesis. Even if Cr levels are unchanged, these results show that loss of Slc6a8 expression in DA neurons leads to persistent hyperactivity, which is especially relevant given the high rate of ADHD in the CTD population. Future studies will look for attentional abnormalities in dCrt−/y mice. Because dCrt−/y mice display a robust hyperactivity, this may indicate the presence of an ADHD-like phenotype and potentially explain the high comorbidity of ADHD and CTD. Future studies will also examine the causes of the weight differences between dCrt−/y and control mice. DA is highly involved in motivation and it is possible that alterations to the dopaminergic system could lead to altered feeding behavior (Palmiter 2007).
Acknowledgments:
The authors would like to thank Marla K. Perna, and Keila N. Miles for providing scientific input and proofreading this article.
Funding: This work was supported by National Institutes of Health grant HD080910 and a CARE grant from the Association of Creatine Deficiencies. Portions were supported by the Kerman Family Fund, the Selma Schottenstein Harris Lab for Research in Parkinson’s, the Gardner Family Center for Parkinson’s Disease and Movement Disorders, and the Parkinson’s Disease Support Network - Ohio, Kentucky and Indiana.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Abdulla ZI, Pennington JL, Gutierrez A and Skelton MR (2019). "Creatine transporter knockout mice (Slc6a8) show increases in serotonin-related proteins and are resilient to learned helplessness." bioRxiv: 641845. [DOI] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H and Tomac AC (2006). "Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus." Genesis 44(8): 383–390. [DOI] [PubMed] [Google Scholar]
- Beninger RJ (1983). "The role of dopamine in locomotor activity and learning." Brain Research Reviews 6: 173–196. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Krieger AM and Yekutieli D (2006). "Adaptive linear step-up procedures that control the false discovery rate." Biometrika 93(3): 491–507. [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K and Wallen-Mackenzie A (2010). "VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation." Proc Natl Acad Sci U S A 107(1): 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Henry H, Loup M, Eilers B and Bachmann C (2001). "Endogenous synthesis and transport of creatine in the rat brain: an in situ hybridization study." Molecular Brain Research 86(1-2): 193–201. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Salomons GS, Ball J. William S., Wong B, Chuck G, Verhoeven NM, Jakobs C and DeGrauw TJ (2001). "Irreversible Brain Creatine Deficiency with Elevated Serum and Urine Creatine: A CreatineTransporter Defect?" Ann Neurol 49: 401–404. [DOI] [PubMed] [Google Scholar]
- Cunha MP, Martin-de-Saavedra MD, Romero A, Egea J, Ludka FK, Tasca CI, Farina M, Rodrigues AL and Lopez MG (2014). "Both creatine and its product phosphocreatine reduce oxidative stress and afford neuroprotection in an in vitro Parkinson's model." ASN Neuro 6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MP, Martin-de-Saavedra MD, Romero A, Parada E, Egea J, Del Barrio L, Rodrigues AL and Lopez MG (2013). "Protective effect of creatine against 6-hydroxydopamine-induced cell death in human neuroblastoma SH-SY5Y cells: Involvement of intracellular signaling pathways." Neuroscience 238: 185–194. [DOI] [PubMed] [Google Scholar]
- deGrauw TJ, Salomons GS, Cecil KM, Chuck G, Newmeyer A, Schapiro MB and Jakobs C (2002). "Congenital Creatine Transporter Deficiency." Neuropediatrics 33: 232–238. [DOI] [PubMed] [Google Scholar]
- Faraone SV (2018). "The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities." Neurosci Biobehav Rev 87: 255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glajch KE, Fleming SM, Surmeier DJ and Osten P (2012). "Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson's disease." Behav Brain Res 230(2): 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Galindo MF, Concannon CG, Segura MF, Fernandez-Gomez FJ, Llecha N, Comella JX, Prehn JH and Jordan J (2008). "6-Hydroxydopamine activates the mitochondrial apoptosis pathway through p38 MAPK-mediated, p53-independent activation of Bax and PUMA." J Neurochem 104(6): 1599–1612. [DOI] [PubMed] [Google Scholar]
- Hautman ER, Kokenge AN, Udobi KC, Williams MT, Vorhees CV and Skelton MR (2014). "Female mice heterozygous for creatine transporter deficiency show moderate cognitive deficits." J Inherit Metab Dis 37(1): 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerle AM, Dickerson JW, Herring NR, Schaefer TL, Vorhees CV, Williams MT and Seroogy KB (2012). "(+/−)3,4-methylenedioxymethamphetamine ("ecstasy") treatment modulates expression of neurotrophins and their receptors in multiple regions of adult rat brain." J Comp Neurol 520(11): 2459–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosamani R, Ramesh SR and Muralidhara (2010). "Attenuation of rotenone-induced mitochondrial oxidative damage and neurotoxicty in Drosophila melanogaster supplemented with creatine." Neurochem Res 35(9): 1402–1412. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Calingasan NY, Starkov A, Stavrovskaya IG, Kristal BS, Yang L, Wieringa B and Beal MF (2004). "Neuroprotective mechanisms of creatine occur in the absence of mitochondrial creatine kinase." Neurobiol Dis 15(3): 610–617. [DOI] [PubMed] [Google Scholar]
- Koob GF, Riley SJ, Smith SC and Robbins TW (1978). "Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat." Journal of Comparative and Physiological Psychology 92(5): 917–927. [DOI] [PubMed] [Google Scholar]
- Lowe MT, Kim EH, Faull RL, Christie DL and Waldvogel HJ (2013). "Dissociated expression of mitochondrial and cytosolic creatine kinases in the human brain: a new perspective on the role of creatine in brain energy metabolism." J Cereb Blood Flow Metab 33(8): 1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Meuller G, Kaddurah-Daouk R and Beal MF (1999). "Creatine and Cyclocreatine Attenuate MPTP Neurotoxicity." Experimental Neurology 157: 142–149. [DOI] [PubMed] [Google Scholar]
- Newmeyer A, Cecil KM, Schapiro M, Clark JF and Degrauw TJ (2005). "Incidence of Brain Creatine Transporter Deficiency in Males with Developmental Delay Referred for Brain Magnetic Resonance Imaging." Developmental and Behavioral Pediatrics 26(4): 276–282. [DOI] [PubMed] [Google Scholar]
- Numan S, Gall CM and Seroogy KB (2005). "Developmental expression of neurotrophins and their receptors in postnatal rat ventral midbrain." J Mol Neurosci 27(2): 245–260. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Tachikawa M, Takanaga H, Shimizu H, Watanabe M, Hosoya K and Terasaki T (2002). "The blood-brain barrier creatine transporter is a major pathway for supplying creatine to the brain." J Cereb Blood Flow Metab 22(11): 1327–1335. [DOI] [PubMed] [Google Scholar]
- Palmiter RD (2007). "Is dopamine a physiologically relevant mediator of feeding behavior?" Trends Neurosci 30(8): 375–381. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Tieu K, Perier C and Vila M (2004). "MPTP as a Mitochondrial Neurotoxic Model of Parkinson’s Disease." Journal of Bioenergetics and Biomembranes 36(4): 375–379. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Zis AP and Fibiger HC (1975). "Ascending catecholamine pathways and amphetamine-induced locomotor activity: Importance of dopamine and apparent non-involvment of norepinephrine." Brain Research 93: 441–454. [DOI] [PubMed] [Google Scholar]
- Rosenberg EH, Almeida LS, Kleefstra T, deGrauw RS, Yntema HG, Bahi N, Moraine C, Ropers H, Fryns J, Degrauw TJ, Jakobs C and Salomons GS (2004). "High Prevalence of SLC6A8 Deficiency in X-Linked Mental Retardation." Am. J. Hum. Genet 75: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runegaard AH, Sorensen AT, Fitzpatrick CM, Jorgensen SH, Petersen AV, Hansen NW, Weikop P, Andreasen JT, Mikkelsen JD, Perrier JF, Woldbye D, Rickhag M, Wortwein G and Gether U (2018). "Locomotor- and Reward-Enhancing Effects of Cocaine Are Differentially Regulated by Chemogenetic Stimulation of Gi-Signaling in Dopaminergic Neurons." eNeuro 5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K and Gash DM (1994). "Effect of bilateral 6-OHDA lesions of the substantia nigra on locomotor activity in the rat." Brain Research 633: 144–150. [DOI] [PubMed] [Google Scholar]
- Seroogy KB and Herman JP (1997). "In situ hybridization approaches to the study of the nervous system" In: Neurochemistry: A Practical Approach (2nd Edition), Turner AJ and Bachelard HS (eds.) Oxford University Press, Oxford: 121–150. [Google Scholar]
- Skelton MR, Schaefer TL, Graham DL, Degrauw TJ, Clark JF, Williams MT and Vorhees CV (2011). "Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency." PLoS One 6(1): e16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein MA, Hu CX, Scicchitano MS, Narayanan PK, McFarland DC, Thomas HC and Schwartz LW (2005). "Effects of 6-hydroxydopamine on mitochondrial function and glutathione status in SH-SY5Y human neuroblastoma cells." Toxicol In Vitro 19(4): 471–479. [DOI] [PubMed] [Google Scholar]
- Udobi KC, Delcimmuto N, Kokenge AN, Abdulla ZI, Perna MK and Skelton MR (2019). "Deletion of the creatine transporter gene in neonatal, but not adult, mice lead to cognitive deficits." bioRxiv: 582320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udobi KC, Kokenge AN, Hautman ER, Ullio G, Coene J, Williams MT, Vorhees CV, Mabondzo A and Skelton MR (2018). "Cognitive deficits and increases in creatine precursors in a brain-specific knockout of the creatine transporter gene Slc6a8." Genes Brain Behav 17(6): e12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullio-Gamboa G, Udobi KC, Dezard S, Perna MK, Miles KN, Costa N, Taran F, Pruvost A, Benoit JP, Skelton MR, Lonlay P and Mabondzo A (2019). "Dodecyl creatine ester-loaded nanoemulsion as a promising therapy for creatine transporter deficiency." Nanomedicine (Lond) 14(12): 1579–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kamp JM, Betsalel OT, Mercimek-Mahmutoglu S, Abulhoul L, Grunewald S, Anselm I, Azzouz H, Bratkovic D, de Brouwer A, Hamel B, Kleefstra T, Yntema H, Campistol J, Vilaseca MA, Cheillan D, D'Hooghe M, Diogo L, Garcia P, Valongo C, Fonseca M, Frints S, Wilcken B, von der Haar S, Meijers-Heijboer HE, Hofstede F, Johnson D, Kant SG, Lion-Francois L, Pitelet G, Longo N, Maat-Kievit JA, Monteiro JP, Munnich A, Muntau AC, Nassogne MC, Osaka H, Ounap K, Pinard JM, Quijano-Roy S, Poggenburg I, Poplawski N, Abdul-Rahman O, Ribes A, Arias A, Yaplito-Lee J, Schulze A, Schwartz CE, Schwenger S, Soares G, Sznajer Y, Valayannopoulos V, Van Esch H, Waltz S, Wamelink MM, Pouwels PJ, Errami A, van der Knaap MS, Jakobs C, Mancini GM and Salomons GS (2013). "Phenotype and genotype in 101 males with X-linked creatine transporter deficiency." J Med Genet 50(7): 463–472. [DOI] [PubMed] [Google Scholar]
- van de Kamp JM, Mancini GM and Salomons GS (2014). "X-linked creatine transporter deficiency: clinical aspects and pathophysiology." J Inherit Metab Dis 37(5): 715–733. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Tokarska-Schlattner M and Schlattner U (2011). "The creatine kinase system and pleiotropic effects of creatine." Amino Acids 40(5): 1271–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M and Kaddurah-Daouk R (2000). "Creatine and Creatinine Metabolism." Physiol Rev 3(80): 1107–1213. [DOI] [PubMed] [Google Scholar]
- Xiong N, Long X, Xiong J, Jia M, Chen C, Huang J, Ghoorah D, Kong X, Lin Z and Wang T (2012). "Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson's disease models." Crit Rev Toxicol 42(7): 613–632. [DOI] [PubMed] [Google Scholar]