FIG 1.
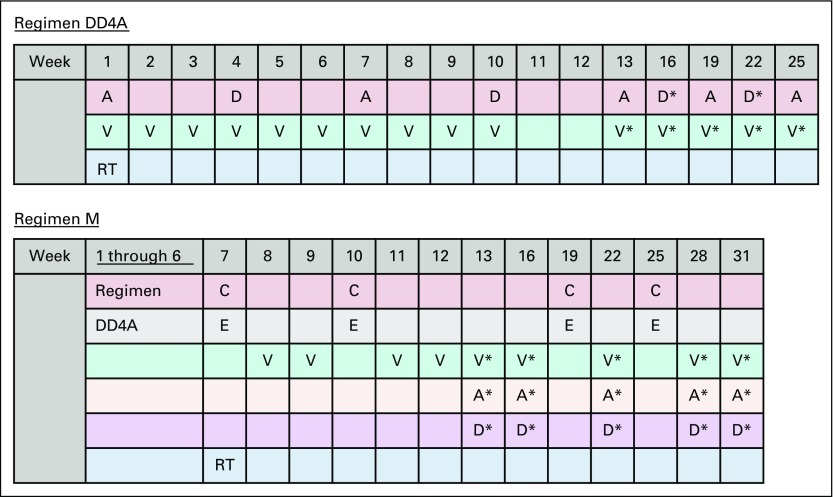
Treatment protocols used in the AREN0532 and AREN0533 studies. Regimen DD4A: vincristine, dactinomycin, and doxorubicin with no radiation therapy (RT); regimen M: vincristine, dactinomycin, and doxorubicin alternating with cyclophosphamide and etoposide with RT. A: dactinomycin 0.023 mg/kg/dose IV × 1 for infants < 1 year; 0.045 mg/kg/dose IV × 1 for children ≥ 1 year (maximum dose: 2.3 mg). C: cyclophosphamide 14.7 mg/kg/dose IV × 5 days for infants < 1 year; 440 mg/m2/dose IV × 5 days for children ≥ 1 year. D: doxorubicin 1.5 mg/kg/dose IV × 1 for infants < 1 year; 45 mg/m2/dose IV × 1 for children ≥ 1 year. D*: doxorubicin 1 mg/kg/dose IV × 1 for infants < 1 year; 30 mg/m2/dose IV × 1 for children ≥ 1 year. E: etoposide 3.3 mg/kg/dose IV × 5 days for infants < 1 year; 100 mg/m2/dose IV × 5 days for children ≥ 1 year. V: vincristine: 0.025 mg/kg/dose intravenously (IV) × 1 for infants < 1 year; 0.05 mg/kg/dose IV × 1 for children ≥ 1 year to 2.99 years; 1.5 mg/m2/dose IV × 1 for children ≥ 3 years (maximum dose: 2 mg). V*: vincristine: 0.034 mg/kg/dose IV × 1 for infants < 1 year; 0.067 mg/kg/dose IV × 1 for children ≥ 1 year to 2.99 years; 2 mg/m2/dose IV × 1 for children ≥ 3 years (maximum dose: 2 mg). RT: for local stage III tumors, 10.8 Gy flank radiation was used, with a 10.8 Gy boost for gross residual disease after surgery. Patients with preoperative tumor rupture, cytology-positive ascites, or diffuse peritoneal seeding were treated with whole-abdomen RT to a dose of 10.5 Gy. Patients with lung metastases received whole lung RT to a dose of 12 Gy in 1.5 Gy fractions (reduced to 10.5 Gy for patients < 12 months old). FHWT, favorable histology Wilms tumor; NWTS-5, National Wilms Tumor Study 5.