Abstract
Biological nitrogen (N) fixation is an important process supporting primary production in ecosystems, especially in those where N availability is limiting growth, such as peatlands and boreal forests. In many peatlands, peat mosses (genus Sphagnum) are the prime ecosystem engineers, and like feather mosses in boreal forests, they are associated with a diverse community of diazotrophs (N2-fixing microorganisms) that live in and on their tissue. The large variation in N2 fixation rates reported in literature remains, however, to be explained. To assess the potential roles of habitat (including nutrient concentration) and species traits (in particular litter decomposability and photosynthetic capacity) on the variability in N2 fixation rates, we compared rates associated with various Sphagnum moss species in a bog, the surrounding forest and a fen in Sweden. We found appreciable variation in N2 fixation rates among moss species and habitats, and showed that both species and habitat conditions strongly influenced N2 fixation. We here show that higher decomposition rates, as explained by lower levels of decomposition-inhibiting compounds, and higher phosphorous (P) levels, are related with higher diazotrophic activity. Combining our findings with those of other studies, we propose a conceptual model in which both species-specific traits of mosses (as related to the trade-off between rapid photosynthesis and resistance to decomposition) and P availability, explain N2 fixation rates. This is expected to result in a tight coupling between P and N cycling in peatlands.
Introduction
Nitrogen (N) fixation is the only biological pathway in which atmospheric dinitrogen (N2) is converted to a reduced form (NHx) accessible to plants. In this way N2-fixing microorganisms (diazotrophs) significantly contribute to the N pools of ecosystems, and thereby affect primary production of plants, given that N availability is often limiting plant biomass production [1]. Knowledge of the drivers of the strongly varying rates of N2 fixation reported in literature is therefore vital for our understanding of the relative contribution of N2 fixation to the total ecosystem N input [2, 3]. Human interference through the development of techniques to artificially fix N2 for agricultural production has led to extensive eutrophication, disrupting the global N cycle [4, 5]. As this disturbance of the natural N input into ecosystems also affects carbon (C) cycling through its effects on primary production and decomposition [6, 7], knowledge about the drivers of N2 fixation becomes even more important.
The relative contribution of N2 fixation to the total available N pool can be expected to be most significant in those ecosystems, such as Sphagnum peatlands, where atmospheric N deposition represents the only other major N input. The low N deposition at northern latitudes, in combination with the competitive strategy of Sphagnum mosses to sequester N, speaks for the importance of N2 fixation in these habitats. In pristine boreal peatlands, which are long term C sinks [8, 9], rates of N2 fixation in Sphagnum were indeed found to explain the discrepancy between the low N inputs through atmospheric deposition, and the N assimilation of Sphagnum species and N storage in peat [10]. In Sphagnum peatlands the contribution of diazotrophic N2 fixation is estimated to constitute around 35% of the N input [11, 12], while in N-limited boreal forests, N2 fixation in mosses was found to contribute up to 50% to the total N input [13].
Peat mosses (genus Sphagnum) are the prime ecosystem engineers of many peatlands, hampering other plants’ growth for example by strongly monopolising N input from atmospheric deposition [14–16]. Also, Sphagnum deters competitors and decomposers through waterlogging, acidification and the production of recalcitrant organic compounds and structurally rigid cells: characteristics that enable them to produce and store large amounts of peat over time [16, 17]. Functional traits of Sphagnum mosses, such as litter decomposability, growth and acidifying potential, are in large part due to the biochemistry of the mosses [18, 19], and their traits affect ecosystem processes such as C sequestration in Sphagnum dominated peatlands [20]. N2 fixation potential represents an important functional trait that contributes to ecosystem N input. In this way plant-associated microbiomes explain additional variation in productivity and ecosystem functioning [21, 22].
Sphagnum mosses are colonised by a diverse community of microorganisms that live inside the large volume of dead hyaline cells and on the surface of their tissue [23, 24]. The community may comprise a high proportion (45.5%; [25]) of highly diverse diazotrophs that profit from the moist and partly anoxic conditions around and in Sphagnum. Other studies have shown that most of the N2-fixing activity in Sphagnum was assigned to the Alphaproteobacterial class of the phylum Proteobacteria, and only very little (6% or less) to the phylum Cyanobacteria [25–27]. The ratio between Proteobacteria and Cyanobacteria can, however, vary among peatlands [28]. This microbiome composition is in stark contrast to that of feather mosses that dominate the forest floor of boreal forests. The feather moss Pleurozium schreberi was associated with diazotrophs whose genes were 96% cyanobacterial [29]. Much research has focused on the differential rates of N2 fixation of cyanobacteria in these feather mosses, and its inhibition by increasing N deposition [29–31]. However, for Sphagnum, much less is known about the variation in N2 fixation rates in different species and habitats, and how environmental drivers control these rates.
Different Sphagnum species were found to have different microbial communities [24], resulting in differences in N2 fixation rates [27]. At the same time, different Sphagnum species are also adapted to the different environmental conditions of their habitats [32, 33], which can also be expected to have an effect on the composition and activity of the diazotrophic community [28]. For example, N2 fixation rates in Sphagnum species of fens were found to be higher than in Sphagnum species in bogs [34]. This may be related to high pH [35] or higher phosphorus (P) availability [36]. In bogs, rates were found to be higher in hollows compared to hummocks [37], suggested to be the result of water level [27]. Especially the effect of P, important for the synthesis of ATP necessary for the costly biochemical process of N2 fixation, is considered to be important in driving N2 fixation rates in terrestrial ecosystems in general [38], and in feather mosses [39] and Sphagnum [36].
Here, we study the variation in N2 fixation rates among host species and habitats, by comparing four Sphagnum mosses in contrasting habitats and relating N2 fixation to nutrient levels in the mosses. Specifically, we relate the N2 fixation to species traits associated with growth and decomposition. Synthesising our results with those of other studies, we generate a conceptual model that illustrates the interconnected drivers of N2 fixation and Sphagnum performance in mires. In addition, we compare the N2 fixation rates in Sphagnum with two well-studied boreal feather mosses.
Material and methods
Species and study sites
Our study sites are located in the central-east of southern Sweden. Kulflyten mire (59°54’N, 15°50’E) is a raised ombrotrophic bog with bog pools, surrounded by a wet lagg fen that is richer in solutes because of the surrounding mineral soil. The mire is further surrounded by a young spruce forest on peaty soil. The second site, Glon (60°31’N, 17°55’E) is a small rich fen on lime-rich moraine. The pH in the bog among our samples averages 4.3 (±0.02), while it is 5.5 (±0.03) around the Sphagnum sampled in the rich fen. Both sites show a mean temperature of around 17°C in July, and –2.6°C (Kulflyten) and –1.0°C (Glon) in December [32]. No specific permissions were required at these sites because Swedish public access to land allows everyone to access nature areas and collect plants, as long as an area does not have a protected status which states that you can not collect plants. These areas did not have protected status prohibiting collection of moss or other plants. The field study did not involve endangered or protected species.
Our sampling aimed to explore the variation among species and habitats. At Kulflyten mire we sampled four different vegetation types: open bog (OB), pine bog (PB), spruce forest (SF) and lagg fen (LF). At the fen, Glon, one habitat type was sampled: rich fen (RF). We sampled four species of Sphagnum: S. fallax (LF), S. fuscum (OB, RF), S. rubellum (OB), S. magellanicum (OB, PB, SF). Additionally, we sampled two species of feather mosses: Pleurozium schreberi (SF) and Hylocomium splendens (SF) (Table 1).
Table 1. Species used in the study, vegetation types and microtopographical positions along the hummock-hollow gradient.
| Species | Author | Sphagnum subgenus | Vegetation type | Micro-topographical position |
|---|---|---|---|---|
| Sphagnum fuscum | (Schimp.) H.Klinggr. | Acutifolia | Open bog (OB) Rich fen (RF) | Hummock Hummock |
| Sphagnum rubellum | Wilson | Acutifolia | Open bog (OB) | Low hummock |
| Sphagnum fallax | (H.Klinggr.) H.Klinggr. | Cuspidata | Lagg fen (LF) | Lawn |
| Sphagnum magellanicum | Brid. | Sphagnum | Open bog (OB) Pine bog (PB) Spruce forest (SF) | Lawn–carpet Hummock Hummock |
| Pleurozium schreberi | (Brid.) Mitt. | - | Spruce forest (SF) | - |
| Hylocomium splendens | (Hedw.) Schimp. | - | Spruce forest (SF) | - |
Sampling and N2 fixation measurements
Samples were collected in September 2014 in homogenous patches of single species. For each species, five replicates (four for S. magellanicum, SF), from similar patches were obtained. For Sphagnum these are the patches where we measured several traits, including photosynthetic capacity, in the previous study [32, 40]. Within each replicate three subsamples were taken. A sharpened metal ring (ø 5 cm, depth 5 cm) was carefully inserted into the vegetation without compressing it, by making a pre-cut circle using scissors. Samples were put in plastic ziplock bags and directly transported to the lab in Nijmegen, the Netherlands. Two of the subsamples were dried for 72 h at 70° C and weighted to determine dry weight (DW) per area and bulk density (g cm-3). These subsamples were homogenised and ground with a mixer mill (MM301, Retsch, Germany) for 2 min at 30 rotations s-1 and used as control samples to determine background isotopic N signature and tissue nutrient concentrations.
The third subsample of each species-habitat combination replicate was put in a 120 ml flask with a capped rubber stopper. With an injection needle, 12 ml of the headspace air was removed and replaced with 15N2 gas (98 atom % 15N, Sigma-Aldrich, Germany) resulting in a headspace concentration of 10% of 15N2. The mosses were incubated for 48h in an incubation room (light regime 16h per day, 200 μmol m-2 s-1 PAR, 20°C). After incubation, mosses were dried and ground (see above). For both background and enriched samples, total N concentrations, isotopic ratios (15N/14N, expressed with δ15N signal as the ‰ deviation from atmospheric N2) and atom percent 15N/14N (15N) were determined using an elemental analyser (Type NA 1500 Carlo Erba, Thermo Fisher Scientific Inc, USA) coupled online via an interface (Finnigan Conflo III) to a mass spectrometer (Thermo Finnigan DeltaPlus, USA). The isotope atom percent 15N of the enriched samples was corrected by the natural isotopic 15N abundance of the background samples by subtracting enriched atom% 15N with natural atom% 15N to get atom% increase in 15N. This atom% increase in 15N is converted to g N gDW-1 with the total %N according to Eq 1.
| (1) |
N2 fixation rates in g N gDW-1 were converted to rates in nmol N2 gDW-1 d-1 using incubation time and molecular weight. Light regime during incubation was representative for the average day length of the growth season (16 hours), and the short period of raising the temperature to 20°C was expected to have little effect on N2 fixation rates [41, 42].
Total carbon (C) concentration was determined using an elemental analyser (see above) and total phosphorus (P) and potassium (K) concentrations were determined on digestates of dried and ground moss tissue, which were prepared by digesting samples in 500 μL HNO3 (65%) and 200 μL H2O2 (30%) for 16 min in a microwave (Milestone MLS1200 Mega, Milestone Inc., Sorisole, Italy). Digestates were diluted with MilliQ water (PFXXXXM1, Elga, UK) and P and K concentrations measured by inductively coupled plasma emission spectrometry (IRIS Intrepid II, Therma Electron corporation, Franklin, USA).
Sphagnum performance and traits
We used data on Sphagnum performance and environmental variables that were collected at the same sample patches and analysed by Bengtsson et al. [32, 40], where methods and data are described in detail. We included data on biomass accumulation per unit area, CO2 exchange rate per unit biomass, decomposition in the field and standardised decomposability in the lab (% mass loss from litter). Biomass accumulation was averaged over the two growing seasons 2013 and 2014. Length increment was measured along brush wires inserted into the Sphagnum vegetation, as described in Rydin and Jeglum [16]. Biomass accumulation was determined by counting the shoot density in cores (ø = 7 cm) and multiplying the DW of 3 cm shoot-sections with the length increment. Photosynthetic capacity was measured as the maximum net CO2 exchange rate at optimal water content and expressed per individual shoot.
Moss decomposability was measured in samples from the same patches used by Bengtsson et al. [19, 43], where methods and data are described in detail. Decomposability of the litter was determined as mass loss after litter had been incubated in the lab for 7 months. Litter was defined as 2 cm shoot sections from below the capitula, which was placed in nylon mesh bags. To estimate field mass loss, a second set of litterbags was buried in the field at the original location of each bag, 5 cm below the moss surface. Mass loss was assessed after 14 months. We also use data for the same Sphagnum patches on biochemical composition of the litter: sphagnan, soluble phenolics and lignin-like phenolics (all in mg g-1).
Statistical analysis
To analyse whether rates of N2 fixation varied between species and habitats we used ANOVA. Samples of S. fuscum and S. magellanicum from different habitats were treated as different units, i.e. on the same level as species when analysing differences between species. Significant differences between groups were tested with a Tukey Post Hoc test.
For the Sphagnum species we also had data on traits, and for these we analysed regressions with N2 fixation rates as the response variable, and decomposability in the lab, decomposition in the field, biomass growth, photosynthetic capacity and tissue element concentrations of biochemical compounds, C, N, P and K (as well as C/N and N/P ratios) as predictors. Residuals were checked for normality and N2 fixation rates were log-transformed (ln(y+1)). To analyse differences in nutrient concentrations, decomposition and growth parameters between samples of S. magellanicum from different habitats a nonparametric Kruskal Wallis test was used. Statistical tests were performed using SPSS Statistics 21.0 [44] and R version 3.4.0 [45].
Results
N2 fixation rates of Sphagnum and feather mosses from different habitats
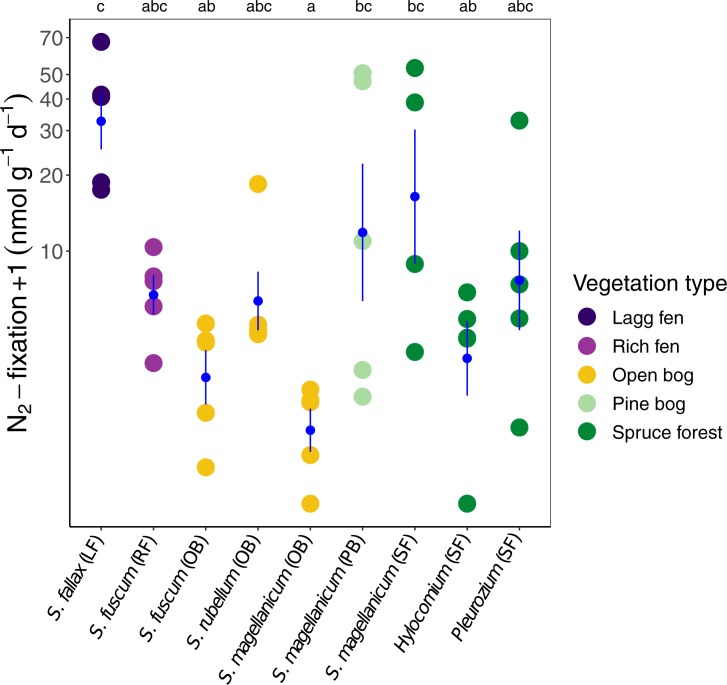
N2 fixation rates varied between Sphagnum species (ANOVA: F8,35 = 5.35; P < 0.001, Fig 1). N2 fixation rates were the highest for S. fallax samples, but the rates of these samples could only be statistically distinguished from S. fuscum (OB), S. magellanicum (OB) and from the feather moss Hylocomium splendens. Compared with all Sphagnum samples, N2 fixation rates in the two feather mosses were at intermediate levels.
Fig 1. N2 fixation rates +1 on a logarithmic scale plotted for Sphagnum and feather moss species and habitats.
Blue shows averages ± SEM (N = 5, except for S. magellanicum (SF) with N = 4). Different letters represent significant differences between species and habitats (based on Tukey tests).
For S. magellanicum, we saw a strong effect of habitat (Fig 1) with N2 fixation averages being more than 20 times higher in the pine bog (PB) and spruce forest (SF), than in the open bog (OB). For this species, there were also significant differences in P concentration between the three habitats (Kruskal Wallis; P < 0.005): lowest P concentrations in OB, followed by PB and highest in SF.
N2 fixation could potentially be affected by microsite wetness (affecting redox potential), but we found no such relationship: the wettest carpet and lawn habitats had samples with both the highest (S. fallax) and lowest (S. magellanicum, open bog) rates (Fig 1), and the hummock samples were intermediate.
Traits related to N2 fixation in Sphagnum
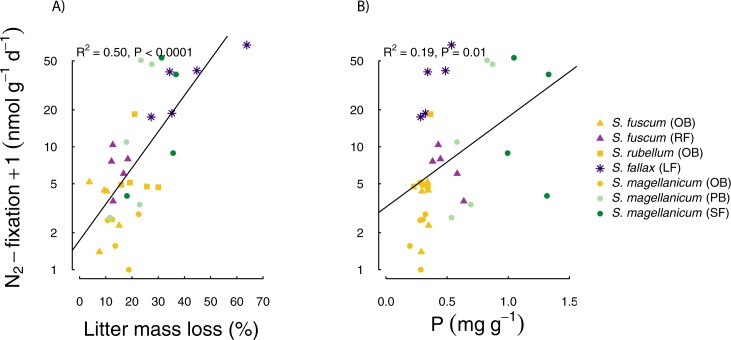
N2 fixation rates for Sphagnum species throughout different habitats showed the strongest significant positive correlations with lab decomposition (r = 0.71; Fig 2A), and in order of weaker correlations with N/P ratio (r = -0.48), lignin-like phenolics (r = -0.46), P concentration (r = 0.43, Fig 2B), field decomposition (r = 0.40) and C concentration (r = -0.39) (Table 2). Photosynthetic capacity was most strongly correlated with P (r = 0.58), N (r = 0.51) and C/N (r = -0.43).
Fig 2.
N2 fixation rate +1 on a logarithmic scale, plotted against A) decomposability (litter mass loss (%) after 7 months incubation in the lab) and B) P concentration (mg g-1) of Sphagnum dry weight.
Table 2. Correlations within the Sphagnum data set.
| Loss lab | Loss field | Biomass growth | Photos. cap | C | N | P | K | N/P ratio | C/N ratio | Sphagnan | Soluble phenolics | Lignin-like phenolics | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nfix log | 0.71** | 0.4* | 0.31 | 0.14 | -0.39* | 0.25 | 0.43* | 0.30 | -0.48** | -0.29 | 0.04 | -0.17 | -0.46** |
| Loss lab | 0.45** | 0.61** | 0.22 | -0.52** | 0.31 | 0.31 | 0.02 | -0.25 | -0.33 | -0.37* | -0.44* | -0.78** | |
| Loss field | 0.32 | 0.05 | -0.24 | 0.47** | 0.15 | 0.22 | -0.02 | -0.5** | -0.06 | -0.08 | -0.49** | ||
| Biomass growth | 0.01 | -0.39* | 0.17 | -0.14 | -0.39* | 0.29 | -0.19 | -0.50** | -0.60** | -0.43* | |||
| Photosynthetic capacity | -0.16 | 0.51** | 0.58** | 0.24 | -0.33 | -0.43* | 0.04 | -0.01 | -0.09 | ||||
| C | -0.27 | -0.19 | 0.02 | -0.04 | 0.28 | 0.33 | 0.69** | 0.59** | |||||
| N | 0.66** | 0.43* | -0.14 | -0.98 | 0.12 | -0.10 | 0.01 | ||||||
| P | 0.60** | -0.77** | -0.63** | 0.19 | 0.01 | -0.06 | |||||||
| K | -0.54** | -0.44** | 0.26 | 0.39* | 0.05 | ||||||||
| N/P ratio | 0.14 | -0.24 | -0.23 | 0.10 | |||||||||
| C/N ratio | -0.13 | 0.10 | 0.01 | ||||||||||
| Sphagnan | 0.44* | 0.53** | |||||||||||
| Soluble phenolics | 0.53** |
Variables are Nfix log = N2 fixation (log transformed (ln(y+1)) (nmol N2 g-1 h-1), Loss lab = decomposability, i.e. mass loss (%) from litter in lab conditions 7 months, Loss field = mass loss (%) from litter in field conditions 14 months, Biomass growth = biomass increase per area, averaged between the growth seasons of 2012 and 2013 (g cm-2), Photosynthetic capacity per unit dry weight (mg g-1 h-1), C, N, P, K = element concentrations (mg g-1) and N/P and C/N = nutrient ratios in Sphagnum tissue, and metabolites in Sphagnum tissue, all in mg g-1: sphagnan concentration, soluble phenolics concentration, and lignin-like phenolics concentration. n = 31–34. *, P < 0.05; **, P < 0.01.
In addition to the simple correlations (Table 2) we tested whether regression models with two predictors could predict N2 fixation better. The best combination (highest R2adj) was with decomposability and N/P ratio as predictors (ln(N2fixation rate +1) = 1.715 + 0.06 decomposability—0.055 N/P; P < 0.0001; R2adj = 0.57; compared to R2adj = 0.52 for the model with decomposability and P, and R2adj = 0.49 for the simple model with decomposability as predictor).
N2 fixation was correlated with the lignin-like phenolics (Table 2), but adding the lignin-like phenolics to the regression model with decomposability did not increase the variation explained (R2adj = 0.47). Testing models with three predictors did not improve on R2adj.
Discussion
Variation in N2 fixation rates
The potential N2 fixation rates we measured in feather mosses were on average 3.5 nmol N2 g-1 d-1 for Hylocomium splendens and 10.5 nmol g-1 d-1 for Pleurozium schreberi. This is within the range measured in boreal forests in Finland by Leppänen et al. [29]. They compared N2 fixation for the same two feather mosses in northern and southern (comparable to the latitude for our sites) habitats and found a northern average of around 27.9 nmol g-1 d-1 for both species, and a southern average of 1.8 nmol g-1 d-1 for both species. Lower rates in the southern sites were suggested to be linked to higher N deposition loads at lower latitudes in Scandinavia (> 0.3 g N m-2 y-1 compared to < 0.2 g N m-2 y-1) [29].
If we compare N2 fixation rates in feather mosses to rates in Sphagnum they are intermediate; the open bog sphagna tend to have lower rates, while mire margin sphagna tend to have higher rates. Higher rates in Sphagnum may be expected because of the more consistently higher water content of Sphagnum, leading to more anoxic and thus more favourable conditions for diazotrophs residing in the hyaline cells of Sphagnum (even though we did not find any relationship between habitat wetness and N2 fixation in the Sphagnum samples). The diazotrophic communities of Sphagnum species, mainly comprising Proteobacteria [25, 27], may be more dependent on wetter conditions to prevent oxygen from down regulating hydrogenase, than cyanobacteria, the main diazotrophs in the studied feather mosses [46]. This may lead to some Sphagnum species with higher and some with lower N2 fixation rates than in the feather mosses.
Studies about N2 fixation often include an up-scaling to potential annual fixation per unit area. Even though this is questionable in our case with measurements at only one occasion, we did some rough comparisons based on literature data. Annual N2 fixation rates are known to vary as a result of temperature and precipitation [46, 47], and are appreciable from June to September in Scandinavia [31, 48]. Assuming a growth season of 200 days [48], rates of annual N2 fixation in Sphagnum species in our study range on average from 6 to 120 mg N m-2 y-1. These rates are lower than results from other European peatlands of 140–280 mg N m-2 y-1 [11, 37], and much lower than 480–6230 mg N m-2 y-1 in boreal bogs in Canada [10]. However, at the moderate atmospheric N deposition rates in the research area (600 mg m-2 y-1; [49], inputs of on average 60 mg m-2 y-1 for Sphagnum species still represent an appreciable contribution.
Drivers of Sphagnum-associated N2 fixation
Our most important finding is that N2 fixation rates of Sphagnum species were best explained by decomposability with an additional effect of phosphorus concentration (as P or N/P-ratio). Sphagna that produce higher amounts of compounds such as sphagnan and phenolics have higher intrinsic decay resistance (lower mass loss in the lab) [19], which was supported by the correlations found here. This may suggest that such compounds directly hamper diazotrophic activity. However, there could also be an indirect effect through decomposition rates and the resulting availability of P.
N2 fixation rates in mires have been found to be higher in wetter habitats [11, 37]. This was reflected by our finding that S. fallax consistently showed the highest rates of N2 fixation. However, S. magellanicum from the open bog, which grows equally wet, had relatively low N2 fixation rates. The large differences within S. magellanicum with lower rates on the bog than in the margin habitats, and overall low rates on the bog, suggest that N2 fixation rates are, at least partly, driven by differences between habitats (open bog versus mire margin). This also supports the old results (based on acetylene reduction assays) that open bogs have low rates of N2 fixation (e.g. [50]).
For S. magellanicum, different habitats were sampled, and we found the highest N2 fixation rates in the spruce forest. Since N2 fixation in Sphagnum is known to be much lower in darker conditions [36, 42], as would be the case under a canopy, light availability cannot explain the differences between open and treed habitats. Trees, assimilating nutrients from deeper soil layers and filtering dry deposition from the atmosphere provide input of nutrients as throughfall or litter [51]. Especially P has been shown to limit N2 fixation [3] and therefore a tree-covered habitat with higher P availability may stimulate N2 fixation rates as indicated for S. magellanicum in treed habitats (Fig 2B). The diazotrophic community of S. magellanicum from different habitats may not only differ in activity, but also in their composition [25]. It would therefore be interesting to know whether there is a direct microbiome link to different habitats [52].
Several variables were correlated with N2 fixation rates, and in the absence of controlled experiments, the mechanisms and directions of cause and effects cannot be fully clarified. Some predictors were also correlated with each other. For example, N2 fixation was negatively correlated with C, but C was also correlated with decomposability (Table 2), and did not improve on a model with decomposability as predictor for N2 fixation.
The positive correlation between N2 fixation and P (and negative with N/P ratio) may indicate that P increases the diazotrophs’ need for N to build up cell structures, instigating them to invest in N2 fixation as a source of additional N. This role of P in N2 fixation has been suggested before [3, 36, 39]. N2 fixation could also lead to more N in the Sphagnum tissue, which may in turn promote decomposition. Sphagnum magellanicum samples from the spruce forest have much higher average P concentrations than other species in our study, and the S. magellanicum samples from the pine bog lie in between (Fig 2B). Thereby, P concentrations in our data reflect different levels of P availability in the different habitats. The higher decomposition rates in more nutrient rich habitats may, in addition, provide a positive feedback on nutrient availability, as they increase nutrient turnover rates.
N2 fixation and Sphagnum growth
We did not find any direct correlations between N2 fixation, photosynthetic capacity and growth (Table 1). Instead, higher Sphagnum growth is related to lower amounts of decay-resisting compounds, in accordance with the trade-off between growth and decomposition we found in a larger data-set [19, 32; of which the current data are a subset]. These previous studies also showed that growth is strongly modulated by, for example, water availability, and this may be more important than any direct effects of photosynthetic capacity or N2 fixation.
The Sphagnum species in our study showed N/P ratios indicating strong to moderate N limitation [53]. Sphagnum magellanicum from the spruce forest had the lowest value (9; indicating strong N limitation) and the same species had the highest value in the open bog (31; P limitation) (S1 Table and S2 Table). Toberman et al. [54] recognised that a key role for P was to drive productivity in peatlands, and these systems have been suggested to be co-limited by N and P, and possibly also by K [55–57]. Fritz et al. [55] showed that addition of P to a pristine bog lead to increased Sphagnum growth without a decrease in tissue N concentration. This may be explained by increased N2 fixation as a result of higher P availability. The importance of P as a regulator of microbial activity is also indicated by the high abundance of microbial genes for P uptake and transport found in Sphagnum and peat [58]. This shows that P, in addition to having a direct stimulating effect on Sphagnum as a nutrient co-limiting growth, can also have an indirect effect on Sphagnum growth through higher fixation of N.
There was a positive correlation between photosynthetic rate and tissue N concentration, which can be a result of higher nutrient input, faster mineralisation rates, higher N2 fixation rates, or a combination of these. The tissue N concentration included the N in the microbiome, so we cannot distinguish between the different sources of N. However, since N2 fixation is an energetically costly process, it is likely that the N being fixed is immediately used for the production of cell structures of diazotrophs (and other microorganisms), before becoming available for Sphagnum [36]. Consequently, in the longer term higher P availability also results in higher N availability, which would stimulate growth and influence the C sequestration function of peatlands. Whether changes in these microbial processes, as affected by P, result in a change in the activity or in the composition of these communities warrants further investigation.
Synthesis: Towards a conceptual model
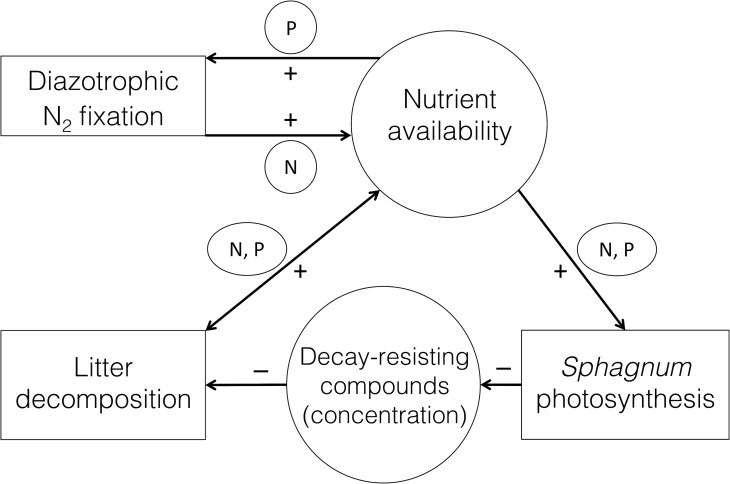
Based on literature and our findings, a conceptual model was generated that summarises the most probable drivers of N2 fixation, photosynthesis and decomposition in Sphagnum, the three processes that we measured in standardised laboratory conditions (Fig 3). This conceptual model may serve to generate testable hypotheses for experimental work in the future.
Fig 3. Conceptual graph showing the potential drivers of N2 fixation, Sphagnum photosynthesis and decomposition.
Arrows indicate an effect (+ positive or–negative) of one process (squares) or reservoir (ellipses) on another, through the availability of nutrients or metabolites.
Sphagnum growth is generally limited by N or P, and in our results photosynthetic capacity was correlated with the concentration of both N and P. In contrast, N2 fixation was correlated with P. Sphagnum species-specific traits are determined by the trade-off between growth and decomposition. Decay rates are related to the concentration of metabolites inhibiting decomposition and to environmental factors such as pH, water table and nutrient availability. We here suggest that faster decomposition can lead to higher P availability (reaching the symbionts by the upward capillary water movement, or by active internal transport; cf. Rydin and Clymo [59]), while higher concentrations of decomposition-inhibiting metabolites may directly or indirectly impede N2 fixation.
Nutrient availability is governed by many processes not depicted in Fig 3 (such as other biological processes, nitrogen deposition, or nutrient inflow into fens and forested ecosystems). We speculate that if a higher input of P to the system (or habitat) stimulates N2 fixation rates, long term Sphagnum growth increases through increased N availability. In conclusion, higher availability of both N and P can be expected to result in increased turnover rates, resulting in a positive feedback loop.
Supporting information
(CSV)
(CSV)
Acknowledgments
We thank Paul van de Ven for assisting with the chemical analyses, Geert Hensgens for his help in the field.
Data Availability
The data are included as Supporting Information files.
Funding Statement
The Swedish Research Council (contract 2015-05174 to HR). The Dutch National Research Organization (‘Cinderella’ ERA-Net project, NWO-grant 861.15.001 to CF). No - The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry. 1991;13(2):87–115. 10.1007/BF00002772 [DOI] [Google Scholar]
- 2.Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, et al. Nitrogen cycles: past, present, and future. Biogeochemistry. 2004;70(2):153–226. [Google Scholar]
- 3.Vitousek PM, Menge DNL, Reed SC, Cleveland CC. Biological nitrogen fixation: rates, patterns and ecological controls in terrestrial ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368(1621):20130119 10.1098/rstb.2013.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canfield DE, Glazer AN, Falkowski PG. The evolution and future of Earth’s nitrogen cycle. Science. 2010;330(6001):192–6. 10.1126/science.1186120 [DOI] [PubMed] [Google Scholar]
- 5.Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, et al. The global nitrogen cycle in the twenty-first century. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368(1621):20130164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bragazza L, Freeman C, Jones T, Rydin H, Limpens J, Fenner N, et al. Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proceedings of the National Academy of Sciences. 2006;103(51):19386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limpens J, Berendse F. How litter quality affects mass loss and N loss from decomposing Sphagnum. Oikos. 2003;103(3):537–47. [Google Scholar]
- 8.Turetsky M, Wieder K, Halsey L, Vitt D. Current disturbance and the diminishing peatland carbon sink. Geophysical Research Letters. 2002;29(11). [Google Scholar]
- 9.Yu Z. Northern peatland carbon stocks and dynamics: a review. Biogeosciences. 2012;9(10):4071. [Google Scholar]
- 10.Vile MA, Wieder RK, Živković T, Scott KD, Vitt DH, Hartsock JA, et al. N2-fixation by methanotrophs sustains carbon and nitrogen accumulation in pristine peatlands. Biogeochemistry. 2014;121(2):317–28. [Google Scholar]
- 11.Larmola T, Leppänen SM, Tuittila ES, Aarva M, Merilä P, Fritze H, et al. Methanotrophy induces nitrogen fixation during peatland development. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):734–9. 10.1073/pnas.1314284111 WOS:000329614500045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg A, Danielsson A, Svensson BH. Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant and Soil. 2013;362(1–2):271–8. 10.1007/s11104-012-1278-4 WOS:000312729400021. [DOI] [Google Scholar]
- 13.Rousk K, Jones DL, DeLuca TH. Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Frontiers in Microbiology. 2013;4 10.3389/fmicb.2013.00150 WOS:000331171800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamers LPM, Bobbink R, Roelofs JGM. Natural nitrogen filter fails in polluted raised bogs. Global Change Biology. 2000;6(5):583–6. 10.1046/j.1365-2486.2000.00342.x WOS:000087864900009. [DOI] [Google Scholar]
- 15.Fritz C, Lamers LPM, Riaz M, van den Berg LJL, Elzenga Tjtm. Sphagnum mosses—masters of efficient N-uptake while avoiding intoxication. PLoS One. 2014;9(1). 10.1371/journal.pone.0079991 WOS:000329866300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rydin H, Jeglum JK. The biology of peatlands, 2e: Oxford University Press; 2013. [Google Scholar]
- 17.Verhoeven J, Liefveld W. The ecological significance of organochemical compounds in Sphagnum. Acta Botanica Neerlandica. 1997;46(2):117–30. [Google Scholar]
- 18.Cornelissen JH, Lang SI, Soudzilovskaia NA, During HJ. Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Annals of Botany. 2007;99(5):987–1001. 10.1093/aob/mcm030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bengtsson F, Rydin H, Hájek T. Biochemical determinants of litter quality in 15 species of Sphagnum. Plant and Soil. 2018;425(1):161–76. 10.1007/s11104-018-3579-8 [DOI] [Google Scholar]
- 20.De Deyn GB, Cornelissen JH, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecology Letters. 2008;11(5):516–31. 10.1111/j.1461-0248.2008.01164.x [DOI] [PubMed] [Google Scholar]
- 21.Laforest-Lapointe I, Paquette A, Messier C, Kembel SW. Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature. 2017;546(7656):145–7. 10.1038/nature22399 [DOI] [PubMed] [Google Scholar]
- 22.Kembel SW, O’Connor TK, Arnold HK, Hubbell SP, Wright SJ, Green JL. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proceedings of the National Academy of Sciences. 2014;111(38):13715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opelt K, Chobot V, Hadacek F, Schonmann S, Eberl L, Berg G. Investigations of the structure and function of bacterial communities associated with Sphagnum mosses. Environmental Microbiology. 2007;9(11):2795–809. 10.1111/j.1462-2920.2007.01391.x WOS:000249990400014. [DOI] [PubMed] [Google Scholar]
- 24.Bragina A, Berg C, Cardinale M, Shcherbakov A, Chebotar V, Berg G. Sphagnum mosses harbour highly specific bacterial diversity during their whole lifecycle. Isme Journal. 2012;6(4):802–13. 10.1038/ismej.2011.151 WOS:000301945500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bragina A, Berg C, Müller H, Moser D, Berg G. Insights into functional bacterial diversity and its effects on Alpine bog ecosystem functioning. Scientific Reports. 2013;3 10.1038/srep01955 WOS:000319960300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kox MAR, Lüke C, Fritz C, van den Elzen E, Alen T, Camp HJM, et al. Effects of nitrogen fertilization on diazotrophic activity of microorganisms associated with Sphagnum magellanicum. Plant and Soil. 2016;406(1):83–100. [Google Scholar]
- 27.Leppänen SM, Rissanen AJ, Tiirola M. Nitrogen fixation in Sphagnum mosses is affected by moss species and water table level. Plant and Soil. 2015;389(1–2):185–96. [Google Scholar]
- 28.Carrell AA, Kolton M, Glass JB, Pelletier DA, Warren MJ, Kostka JE, et al. Experimental warming alters the community composition, diversity, and N2 fixation activity of peat moss (Sphagnum fallax) microbiomes. Global Change Biology. 2019;25(9):2993–3004. 10.1111/gcb.14715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leppänen SM, Salemaa M, Smolander A, Mäkipää R, Tiirola M. Nitrogen fixation and methanotrophy in forest mosses along a N deposition gradient. Environmental and Experimental Botany. 2013;90:62–9. [Google Scholar]
- 30.Ackermann K, Zackrisson O, Rousk J, Jones DL, DeLuca TH. N2 fixation in feather mosses is a sensitive indicator of N deposition in boreal forests. Ecosystems. 2012;15(6):986–98. 10.1007/s10021-012-9562-y WOS:000307763700010. [DOI] [Google Scholar]
- 31.Zackrisson O, DeLuca TH, Gentili F, Sellstedt A, Jäderlund A. Nitrogen fixation in mixed Hylocomium splendens moss communities. Oecologia. 2009;160(2):309–19. 10.1007/s00442-009-1299-8 WOS:000266010700011. [DOI] [PubMed] [Google Scholar]
- 32.Bengtsson F, Granath G, Rydin H. Photosynthesis, growth, and decay traits in Sphagnum—a multispecies comparison. Ecology and Evolution. 2016;6:3325–41. 10.1002/ece3.2119 PMC4833502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rydin H, Jeglum JK. The biology of peatlands. 2 ed Oxford, UK: Oxford University Press; 2013. 432 p. [Google Scholar]
- 34.Waughman GJ, Bellamy DJ. Nitrogen fixation and the nitrogen balance in peatland ecosystems. Ecology. 1980;61(5):1185–98. 10.2307/1936837 WOS:A1980KY31700023. [DOI] [Google Scholar]
- 35.Basilier K. Moss-associated nitrogen fixation in some mire and coniferous forest environments around Uppsala, Sweden. Lindbergia. 1979:84–8. [Google Scholar]
- 36.van den Elzen E, Kox MAR, Harpenslager SF, Hensgens G, Fritz C, Jetten MSM, et al. Symbiosis revisited: phosphorus and acid buffering stimulate N2 fixation but not Sphagnum growth. Biogeosciences. 2017;14(5):1111–22. 10.5194/bg-14-1111-2017 WOS:000396169300001. [DOI] [Google Scholar]
- 37.Granhall U, Selander H. Nitrogen fixation in a subarctic mire. Oikos. 1973;24(1):8–15. [Google Scholar]
- 38.Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry. 2002;57(1):1–45. [Google Scholar]
- 39.Rousk K, Degboe J, Michelsen A, Bradley R, Bellenger JP. Molybdenum and phosphorus limitation of moss‐associated nitrogen fixation in boreal ecosystems. New Phytologist. 2017;214(1):97–107. 10.1111/nph.14331 [DOI] [PubMed] [Google Scholar]
- 40.Bengtsson F, Granath G, Rydin H. Data from: Photosynthesis, growth, and decay traits in Sphagnum–a multispecies comparison. Dryad Digital Repository. 2016. 10.5061/dryad.62054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rousk K, Pedersen PA, Dyrnum K, Michelsen A. The interactive effects of temperature and moisture on nitrogen fixation in two temperate-arctic mosses. Theoretical and Experimental Plant Physiology. 2017;29(1):25–36. [Google Scholar]
- 42.Basilier K, Granhall U, Stenström T-A. Nitrogen fixation in wet minerotrophic moss communities of a subarctic mire. Oikos. 1978:236–46. [Google Scholar]
- 43.Bengtsson F, Rydin H, Hájek T. Data from: Biochemical determinants of litter quality in 15 species of Sphagnum. Dryad Digital Repository. 2018. 10.5061/dryad.4f8d2. [DOI] [Google Scholar]
- 44.IBM Corporation. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corporation; 2012. [Google Scholar]
- 45.R Core Team. R: A language and environment for statistical computing. R version 3.4.0. URL: https://www.R-project.org/. Vienna, Austria: Foundation for Statistical Computing; 2017. [Google Scholar]
- 46.Markham JH. Variation in moss-associated nitrogen fixation in boreal forest stands. Oecologia. 2009;161(2):353–9. 10.1007/s00442-009-1391-0 WOS:000268548000013. [DOI] [PubMed] [Google Scholar]
- 47.Gundale MJ, Wardle DA, Nilsson M-C. The effect of altered macroclimate on N-fixation by boreal feather mosses. Biology Letters. 2012:rsbl20120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeLuca TH, Zackrisson O, Nilsson MC, Sellstedt A. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature. 2002;419(6910):917–20. 10.1038/nature01051 WOS:000178909700041. [DOI] [PubMed] [Google Scholar]
- 49.Granath G, Strengbom J, Breeuwer A, Heijmans MM, Berendse F, Rydin H. Photosynthetic performance in Sphagnum transplanted along a latitudinal nitrogen deposition gradient. Oecologia. 2009;159(4):705–15. 10.1007/s00442-008-1261-1 [DOI] [PubMed] [Google Scholar]
- 50.Basilier K. Moss-associated nitrogen fixation in some mire and coniferous forest environments around Uppsala, Sweden. Lindbergia. 1979;5:84–8. [Google Scholar]
- 51.Aerts R, Verhoeven JTA, Whigham DF. Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology. 1999;80(7):2170–81. [Google Scholar]
- 52.Kyrkjeeide MO, Hassel K, Flatberg KI, Shaw AJ, Yousefi N, Stenøien HK. Spatial genetic structure of the abundant and widespread peatmoss Sphagnum magellanicum Brid. PLoS One. 2016;11(2):e0148447 10.1371/journal.pone.0148447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Güsewell SN. P ratios in terrestrial plants: variation and functional significance. New Phytologist. 2004;164(2):243–66. 10.1111/j.1469-8137.2004.01192.x [DOI] [PubMed] [Google Scholar]
- 54.Toberman H, Tipping E, Boyle JF, Helliwell RC, Lilly A, Henrys PA. Dependence of ombrotrophic peat nitrogen on phosphorus and climate. Biogeochemistry. 2015;125(1):11–20. [Google Scholar]
- 55.Fritz C, Van Dijk G, Smolders AJP, Pancotto VA, Elzenga TJTM, Roelofs JGM, et al. Nutrient additions in pristine Patagonian Sphagnum bog vegetation: can phosphorus addition alleviate (the effects of) increased nitrogen loads. Plant Biology. 2012;14(3):491–9. 10.1111/j.1438-8677.2011.00527.x [DOI] [PubMed] [Google Scholar]
- 56.Wang M, Moore TR. Carbon, nitrogen, phosphorus, and potassium stoichiometry in an ombrotrophic peatland reflects plant functional type. Ecosystems. 2014;17(4):673–84. [Google Scholar]
- 57.Bragazza L, Tahvanainen T, Kutnar L, Rydin H, Limpens J, Hajek M, et al. Nutritional constraints in ombrotrophic Sphagnum plants under increasing atmospheric nitrogen deposition in Europe. New Phytologist. 2004;163(3):609–16. 10.1111/j.1469-8137.2004.01154.x WOS:000223056600018. [DOI] [PubMed] [Google Scholar]
- 58.Lin X, Tfaily MM, Green SJ, Steinweg JM, Chanton P, Imvittaya A, et al. Microbial metabolic potential for carbon degradation and nutrient (nitrogen and phosphorus) acquisition in an ombrotrophic peatland. Applied and Environmental Microbiology. 2014;80(11):3531–40. 10.1128/AEM.00206-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rydin H, Clymo RS. Transport of carbon and phosphorus compounds about Sphagnum. Proceedings of the Royal Society of London Series B, Biological Sciences. 1989;237(1286):63–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(CSV)
Data Availability Statement
The data are included as Supporting Information files.