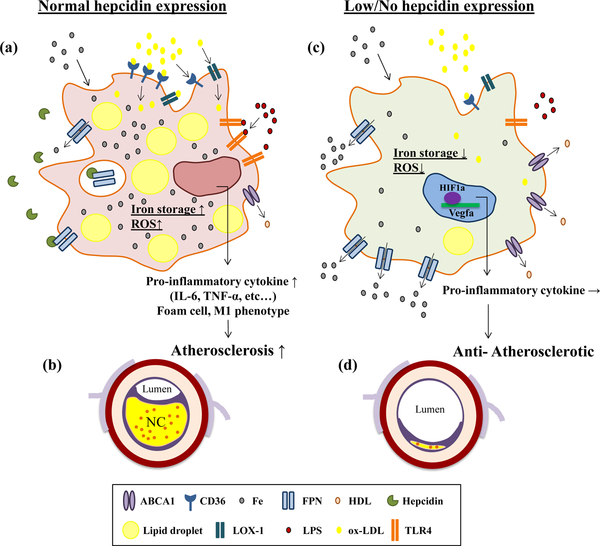
Figure 1. Effect of Hepcidin on Macrophages Phenotype and Function in the Setting of Atherosclerosis.
(a): Hepcidin inhibits iron release from macrophage via down-regulation of iron transporter ferroportin (FPN) and enhance intra-cellular iron storage. Elevated intra-cellular iron accumulation results in incorporation increased oxidized low-density lipoprotein cholesterol (ox-LDL) retention via transporter such as CD36 and LDL recepter-1 (LOX-1), and increased inflammatory signaling (LPS) via toll-like recepter-4 (TLR4), decreasing cholesterol efflux, and intra-cellular reactive oxygen species (ROS) generation. Overall these cells exhibit a phenotype consistent with pro-inflammatory foamy macrophage phenotype. In this condition, macrophages contribute to atherosclerosis progression (b).
(c): In the low/no hepcidin environment, intra-cellular iron is actively exported out of the macrophage via FPN. Lowering intracellular iron within the macrophage suppresses LDL uptake and increases its export via ABC transporters, lowers TLR4-dependent inflammatory signaling and ROS production. These effects are thought to be anti-atherogenic (d). At the same time low iron level enhance hypoxia inducible factor-1 alpha (HIF-1α) nuclear translocation, promoting vascular endothelial target gene expression, endothelial permeability, and inflammatory signaling. The latter effect may be pro-atherogenic especially in the setting of intraplaque hemorrhage.
Abbreviations: ABCA1, ATP-binding cassette sub-family A member 1; CD, cluster differentiation; Fe, iron; FPN, ferroportin; HIF1α, Hypoxia inducible factor-1 alpha; HDL, high-density lipoprotein; LOX-1, low-density lipoprotein recepter-1; LPS, lipopolysaccharide; NC, necrotic core; ox-LDL, oxidized low-density lipoprotein cholesterol; ROS, reactive oxygen species; TLR4, tall-like receptor; TNF-α, tumor necrosis factor-alpha; VEGF-α, vascular endothelial grows factor-alpha.