Summary
Nociceptin and its receptor are widely distributed throughout the brain in regions associated with reward behavior, yet how and when they act is unknown. Here we dissected the role of a nociceptin peptide circuit in reward seeking. We generated a prepronociceptin (Pnoc)-Cre mouse line that revealed a unique subpopulation of paranigral ventral tegmental area (pnVTA) neurons enriched in prepronociceptin. Fiber photometry recordings during progressive ratio operant behavior revealed pnVTAPnoc neurons become most active when mice stop seeking natural rewards. Selective pnVTAPnoc neuron ablation, inhibition and conditional VTA nociceptin receptor (NOPR) deletion increased operant responding, revealing that the pnVTAPnoc nucleus and VTA NOPR signaling are necessary for regulating reward motivation. Additionally, optogenetic and chemogenetic activation of this pnVTAPnoc nucleus caused avoidance and decreased motivation for rewards. These findings provide insight into neuromodulatory circuits that regulate motivated behaviors through identification of a previously unknown neuropeptide-containing pnVTA nucleus that limits motivation for rewards.
Graphical Abstract
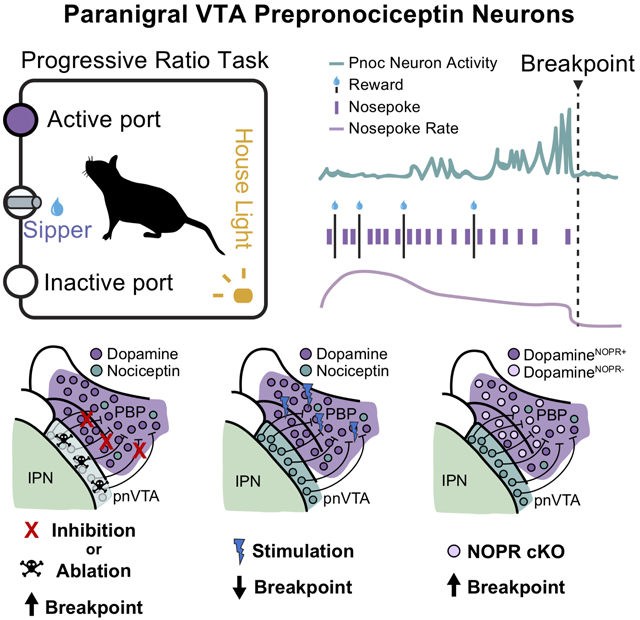
In Brief
A subnuclei of the VTA that expresses the neuropeptide prepronociceptin functions to constrain motivation when the effort to obtain a reward increases
Introduction
Neural circuits that underlie reward seeking are regulated by homeostatic states and adapt to reward availability. Animals are less motivated to seek rewards in environments where resources are scarce (Salamone et al., 2009; Kobayashi and Schultz, 2008). Persistence to seek uncertain rewards can be disadvantageous due to risky exposure to predators or from energy expenditure. To prevent aberrant seeking behavior, neural mechanisms constrain motivation to seek rewards. In humans, deficits within these regulatory processes can manifest into behavioral dysfunctions including depression, addiction, and feeding disorders (Russo and Nestler, 2013; Volkow et al., 2011). In particular, disruption in dopaminergic activity is implicated in the dysregulation of reward seeking and consumption (Morales and Margolis, 2017; Stauffer et al., 2016; Salamone et al., 2009). Evidence suggests heterogeneity of neuronal subtypes and anatomical localization in the VTA, including transmitter and neuropeptide systems that can modulate dopaminergic outputs (Morales and Margolis, 2017; van Zessen et al., 2012; Tan et al., 2012; Jhou et al., 2009). However, how specific neuropeptides regulate motivation through the VTA remains an underexplored area.
Efforts to understand the anatomical basis and specificity of reward regulation by neuropeptides has provided converging evidence that presents the nociceptin (N/OFQ) / nociceptin opioid peptide receptor (NOPR) system as a reward-related neuropeptide system (Castro and Bruchas, 2019; Der-Avakian et al., 2017; Toll et al., 2016; Ozawa et al., 2015; Norton et al., 2002; Zheng et al., 2002; Mollereau and Mouledous, 2000; Mollereau et al., 1994). This opioid system has been proposed to have an endogenous role in regulating motivational state; however, how this regulation may occur remains unresolved. NOPR is broadly expressed in tyrosine hydroxylase (TH)-positive neurons (Norton et al., 2002) and NOPR activation negatively regulates DA neuron activity (Zheng et al., 2002), yet the behavioral consequences of this neuropeptide system’s impact in the VTA are unknown. Anatomical studies highlight this system spans multiple reward-related brain nuclei including nucleus accumbens (NAc), striatum, bed nucleus of stria terminalis (BNST), central amygdala (CeA), hypothalamus, hippocampus, and the VTA (Maidment et al., 2002; Ciccocioppo et al., 2000; Mollereau and Mouledous, 2000; Darland et al., 1998; Anton et al., 1996). Pharmacological evidence has suggested that NOPR stimulation can reduce reward-related behaviors, including food consumption and preference for drugs of abuse (Witkin et al., 2014; Zaveri, 2011; Zhao et al, 2003; Kotlinska et al, 2002; Ciccocioppo et al., 2000). Thus, understanding the mechanisms by which this neuropeptide system modulates reward behavior provides essential circuit-based mechanisms for endogenous VTA regulation, and how opioid peptides in this critical structure impact motivation.
To address a possible link between endogenous nociceptin systems and regulation of reward and motivation, we determined if VTA-projecting neurons expressing the precursor gene for nociceptin, prepronociceptin (Pnoc), are able to gate reward-related behaviors. First, we generated a prepronociceptin (Pnoc)-IRES-Cre knock-in mouse line, implemented retrograde tracing, and identified an enriched Pnoc+ neuronal nucleus within the border of the ventral VTA and interpeduncular nucleus (pnVTA) that projects into lateral portions of the VTA. Neural activity recordings revealed that VTA nociceptin neuron activity increases as mice reduce their effort to obtain rewards. This led to the hypothesis that pnVTAPnoc neurons may regulate motivation for rewards, and that the pnVTAPnoc neurons could bi-directionally alter motivation to seek rewards. Deletion and inhibition of pnVTAPnoc neurons dramatically increased breakpoints to obtain reward whereas optogenetic and chemogenetic stimulation of VTA Pnoc inputs decreased the breakpoints for rewards. We also report that reward seeking is reduced by nociceptin release and NOPR’s action specifically on VTA DA neurons, while the deletion of VTA NOPR or DA neuron NOPR increased reward seeking. Collectively, our findings demonstrate a previously unknown population of pnVTAPnoc neurons positioned to constrain motivation for rewards via specific neuropeptide signaling on DA neurons in the VTA. The identification and interrogation of this pnVTA nociceptin system provides critical insight into the cell types that limit and regulate motivated behavior.
Results
Anatomical Identification of Endogenous Pnoc-expressing VTA Inputs
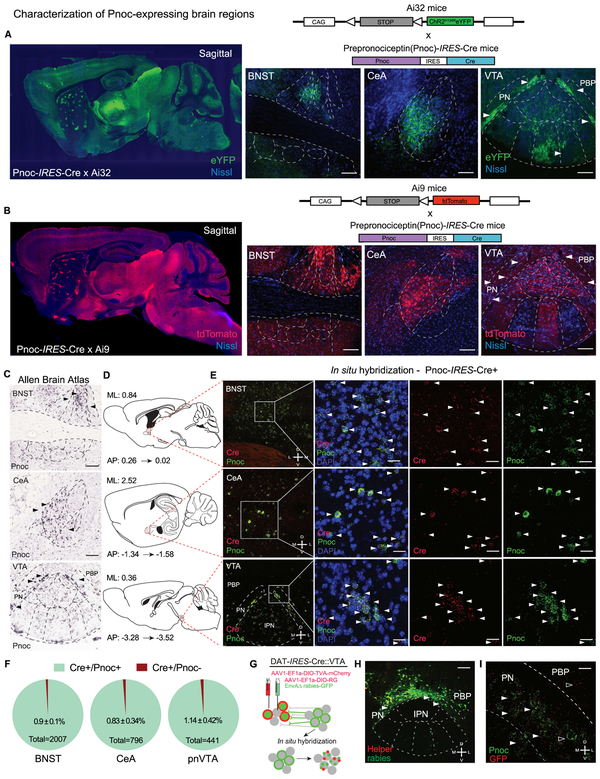
To virally target nociceptin-containing neurons that project into the VTA, we generated a prepronociceptin-IRES-Cre mouse (Pnoc-Cre). A targeted knock-in was used to generate a bicistronic transcript with an IRES to conserve Pnoc expression while providing Cre expression under control of the endogenous locus (see Taniguchi et al., 2011) (Figure S1A). We crossed Pnoc-Cre mice to the tdTomato flox-stop reporter line (Ai9 and Ai14) (Madisen et al., 2010) as well as the channelrhodopsin2-eYFP reporter line (Ai32) (Figures 1A-B, see Methods). These Pnoc-CretdTomato and Pnoc-CreChR2/eYFP lines provided a map for Pnoc-expressing cells and axonal projections. Using these new mouse lines, we observed an abundance of Pnoc-containing cells within the VTA, BNST, CeA, septum, and DG (Figures 1C-E, S1C-D), demonstrating concordance with prior ISH data. We next validated the fidelity of the Pnoc-Cre mouse line by fluorescent in situ hybridization (FISH) co-labeling of Pnoc and Cre in known Pnoc-enriched regions. We observed strong Cre-fidelity in the Pnoc-Cre mouse line, where FISH shows that Pnoc and Cre substantial overlap in Pnoc+ mice in multiple brain areas (BNST, CeA, and VTA), whereas Cre is absent in wildtype littermate mice (Figure S1C). While the majority of these brain regions project to the VTA, it was not known if these projections are specifically Pnoc-expressing.
Figure 1: Anatomical Identification of Endogenous Pnoc-expressing VTA Inputs.
(A-B) Generation of the Pnoc-CretdTomato and Pnoc-CreChR2/eYFP mouse lines from the cross between the Pnoc-Cre × Ai9-tdTomato or Pnoc-Cre × Ai32-ChR2/eYFP. Sagittal images of Pnoc labeling in Pnoc-Cre × Ai9 or Ai32 with coronal images depicting bed nucleus of stria terminalis (BNST), central amygdala (CeA), and paranigral ventral tegmental area (pnVTA). Images show tdTomato (red) or ChR2/eYFP (green) and Nissl (blue) staining. Scale bars are 100 μm.
(C) Representative coronal images for Pnoc in situ hybridization from Allen Brain Institute of the BNST, CeA, VTA/IPN regions. All scale bars are 100 μm.
(D) Sagittal atlas images depicting the location of BNST, CeA, and VTA regions used for quantification.
(E) Pnoc and Cre expression patterns in Pnoc-Cre+ mice via in situ hybridization of Pnoc (green), Cre (red), and DAPI (blue) for coronal images of BNST, CeA, and pnVTA regions corresponding to panel D. Scale bars are 25 μm.
(F) Quantification of Pnoc expression within Cre-expressing cells in the BNST, CeA, and pnVTA (n=4, 4 mice, 3 slices each)
(G) Schematic depicting in situ hybridization for GFP (red) and Pnoc (green) mRNA following recombinant AAV helper viral and rabies viral injections into the VTA of DAT-Cre mice.
(H) Coronal image depicting cre-dependent helper (red) and monosynaptic rabies (green) viral expression in the VTA / IPN regions of DAT-Cre mice. PBP- parabrachial pigmented nucleus, PN – paranigral VTA. Scale bars are 200 μm.
(I) Coronal image depicting fluorescent in situ hybridization (FISH) of Pnoc (green) and GFP (red) colocalization in the pnVTA of DAT-Cre mice following recombinant AAV helper viral and rabies viral injections into the VTA. Scale bars are 25 μm.
To identify putative Pnoc+ VTA-projecting brain regions we utilized two retrograde tracing approaches. First, to survey potential projections interacting with DA neurons, we injected a mixture of AAV1-EF1α-DIO-TVA-mCherry: AAV1-EF1α-DIO-RG into the lateral VTA of DAT-Cre mice followed by an injection SADΔG-GFP (EnvA, rabies) 20 days later (Tian et al., 2016; Watabe-Uchida et al., 2012). Here, we identified multiple sources of monosynaptic inputs onto VTA DA neurons. Of particular interest was the dense labeling in ventral portions of the VTA, particularly in the paraintrafasciular (PIF) and paranigral nucleus (PN) (Figure 1F, S1F). This area showed robust GFP labeling without helper virus (mCherry), suggesting that local VTA Pnoc cells may connect directly onto VTA DA neurons as we observed robust Pnoc expression patterns within the paranigral VTA (Figure 1A-C). To confirm this observation, we performed in situ hybridization for Pnoc and GFP following this rabies tracing approach (Figure 1F). Here we visualized GFP and Pnoc co-labeling in the pnVTA (Figure 1E, S1G) suggesting Pnoc-expressing neurons have synaptic inputs onto DA (DAT-Cre+) cells. To further examine connectivity of this nucleus with the VTA, we injected a retroAAV2-EF1α-DIO-hChR2-(H134R)-eYFP (Tervo et al., 2016), into the lateral VTA of Pnoc-Cre mice and visualized eYFP-labeled ChR2+ expressing neurons within the pnVTA. Additionally, we performed in situ hybridization for Pnoc and DAT and found that the majority of pnVTAPnoc neurons do not express DAT (Figure S1J-K). Together, these data support the conclusion that Pnoc-expressing neurons in the pnVTA synapse onto VTA DA neurons.
Pnoc+ VTA Inputs are Engaged During Low-Yield Reward-Seeking
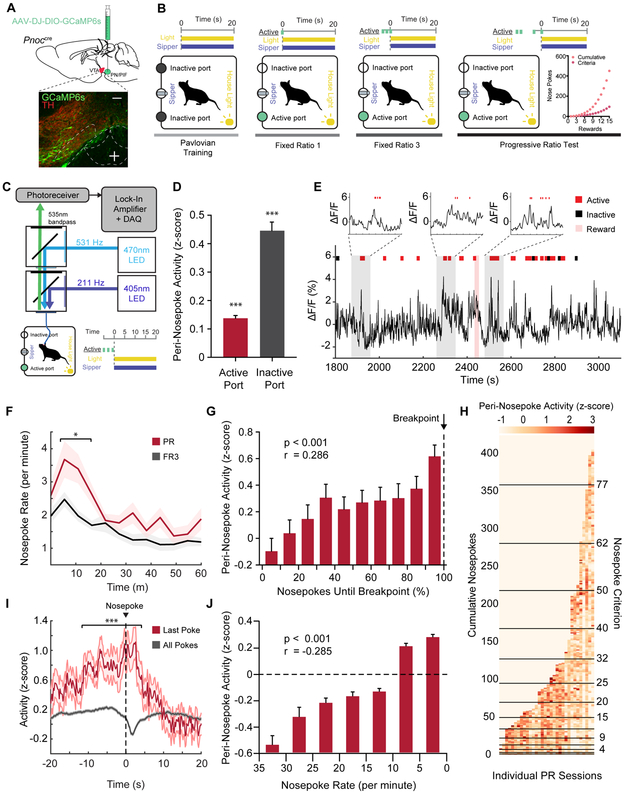
To determine if VTAPnoc neurons are active during reward seeking, we recorded the intracellular calcium dynamics (Pologruto et al., 2004) of VTAPnoc neurons and their terminals in the VTA. We used fiber photometry (Gunaydin et al., 2014) while mice performed an operant task to receive sucrose reward (Figure 2B, 2C). Mice were injected with 400 nl of AAV-DJ-EF1α-DIO-GCaMP6s unilaterally into the pnVTA and implanted with a 400mm optical fiber in the VTA (Figure 2A). Mice were food restricted and underwent Pavlovian conditioning to associate a house light (CS) with access to a sucrose sipper (US). Mice were trained on fixed ratio (FR) operant schedules to nosepoke for 10% sucrose under an FR1 and then under a FR3 schedule. Following FR3 testing, mice were put through a progressive ratio (PR) test, a task that directly measures the breakpoint at which an animal is unwilling to further work for reward (Hodos, 1961; Richardson and Roberts, 1996). Here, nosepoke criterion exponentially increases for each subsequent reward during the task. Neural activity was recorded during the final FR3 day and the PR test day for two training cycles. Typical baseline drift was observed over the hour-long recording sessions and corrected using standard methods (Parker et al., 2016; Gunaydin et al., 2014) (Figure S2A-C). We found that Pnoc+ neural activity in the VTA increased phasically when mice were nosepoking in both FR3 and PR tasks (Figure 2E). Mice nosepoke at a consistent rate throughout the FR3 task, yet in the PR task, mice initially poke at a higher rate, and progressively reduce their poke rate as rewards became more difficult to attain (Figure 2F). Further, evidence shows that VTA DA cells are engaged during operant reward seeking and DA cells are particularly active when effort is high-yield and rewards are attained at a high rate (Gan, Walton, and Phillips, 2010; Hamid et al., 2016; Oliva and Wanat, 2019). To determine how pnVTAPnoc neurons are active during low and high-yield reward seeking, we analyzed Pnoc+ neural activity during nosepoke epochs for different states of PR task performance. To quantify nosepoke-aligned Pnoc+ neural activity, mean fluorescence was assessed in 2-second time bins centered on nosepoke events. All peri-nosepoke activity is shown in Figure 2H for individual PR sessions, where each colored pixel represents mean neural activity for an individual active nosepoke. Peri-nosepoke activity was consistently above baseline for nosepokes into the active and inactive ports for the FR3 and PR tasks (Figure 2D). Assessing nosepoke-aligned activity during PR task performance revealed that activity was initially low during nosepokes early in the PR task when rewards were readily attainable. However, activity increased later in the PR session as more nosepokes were required for each subsequent reward. Interestingly, activity was highest during animals’ final nosepokes before reaching breakpoint and ceasing reward seeking (Figure 2G, 2I, S2F). Activity was also examined relative to the rate of nosepoking. Nosepoke rate was calculated as the total number of active nosepokes occurring within a 1-minute window centered around a given nosepoke event. Peri-nosepoke activity was at its minimum during high rates of nosepoking when mice were seeking rewards vigorously and increased as the poke rate diminished throughout the PR session (Figure 2J). Altogether, these findings indicate that Pnoc+ VTA inputs are engaged during reward seeking, and this activity is negatively correlated to the value of effort. Notably, Pnoc+ neural activity returned to baseline several seconds after an animal ceases its reward-seeking behavior (Figure 2I). These data support the conclusion that VTA Pnoc+ neurons are engaged during low-yield reward seeking, rather than active in the absence of reward anticipation.
Figure 2: Pnoc+ VTA Inputs are Engaged During Low-Yield Reward Seeking.
(A) Sagittal brain cartoon (top panel) of viral injection of GCaMP6s into the pnVTA of Pnoc-Cre+ mice and representative coronal image (bottom panel) showing immunohistochemistry for GCaMP6s and tyrosine hydroxylase (TH) staining. Images show GCaMP6s (green), and TH (red). Scale bars are 100 μm.
(B) Cartoon schematic depicting training regimen for Pavlovian conditioning and operant task within operant box.
(C) Cartoon schematic of fiber photometry setup for recording from mouse within operant box.
(D)Neural activity during 2 second time bins centered around active or inactive nosepoke events (n = 18 mice, 65 sessions: One sample t-test, ***p<0.001).
(E) Representative recorded activity during PR operant task (red ticks = active nosepoke, black ticks = inactive nosepoke, red highlight = 20s reward period).
(F) Mean nosepoke rate (active nosepokes per minute) over entire hour-long FR3 sessions and PR sessions (n = 18 mice, 65 sessions: Two sample t-test, *p=0.031).
(G) Mean peri-nosepoke activity relative to proportion of nosepokes until breakpoint for each PR session (n = 18 mice, 32 sessions: Pearson’s correlation on uncategorized data points, ***p<0.001, r = 0.286).
(H) Individual 2s time bins of mean neural activity centered around active nosepoke events; shown for every active nosepoke during PR sessions (n = 18 mice, 32 sessions). Horizontal black lines indicate attainment of reward for given PR level.
(I) Mean neural activity aligned to nosepoke times for all active nosepokes and the last active nosepoke (breakpoint) for PR sessions (n = 18 mice, 32 sessions: Two sample t-test, ***p<0.001).
(J) Mean peri-nosepoke activity relative to nosepoke rate (active nosepokes per minute) (n = 18 mice, 32 sessions: Pearson’s correlation on uncategorized data points, ***p<0.001, r = −0.285).
Distinct Populations of VTAPnoc Neurons are Engaged During Reward Consumption
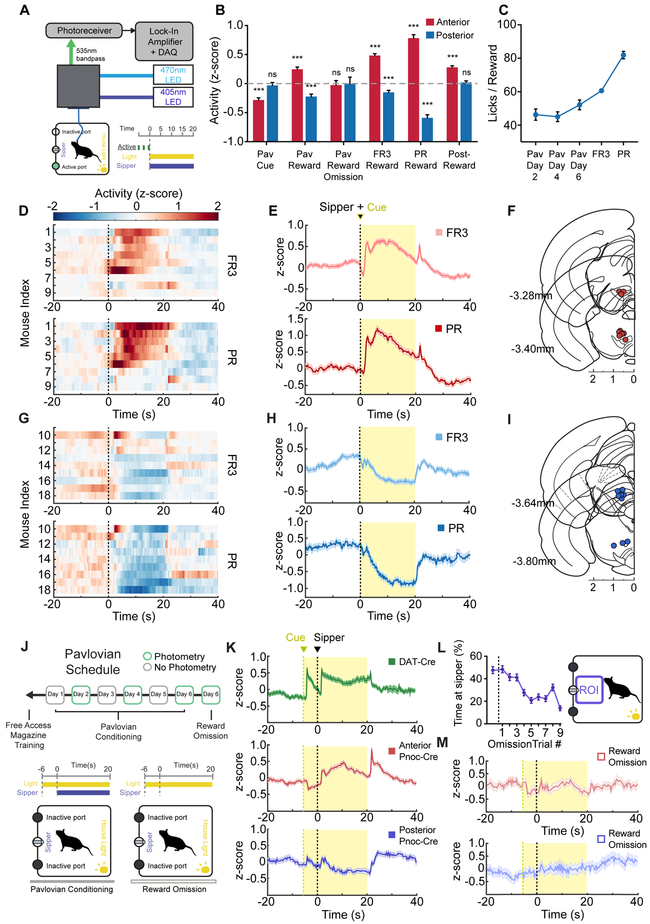
We also analyzed the activity of VTAPnoc neurons during other behavioral epochs to offer a comprehensive evaluation of these unique neurons’ responsivity. We found a divergence in VTA Pnoc+ neural activity during reward consumption between two distinct groups of mice (Figure 3D and 3G). Analysis of optical fiber placement with matched recordings revealed anatomically distinct neural activity during consumption. Mice with anterior optical fibers implanted over pnVTA (anterior to −3.5 AP) had increased activity during reward consumption in FR3 and PR tasks (Figure 3E and 3F) while mice implanted in posterior VTA (posterior to −3.5 AP) had decreased neural activity during reward consumption in FR3 and PR tasks (Figure 3H and 3I). However, analysis revealed that Pnoc+ VTA inputs for both groups of mice had increased activity as mice reduced their rate of reward seeking (Figure 2G and 2J) and that there was no significant effect of fiber placement location and PR task progress in determining peri-nosepoke activity (Figure S2E). This suggests VTAPnoc neurons and their fiber terminals in the anterior and posterior VTA are engaged similarly during nosepoking for reward but diverge in their neural activity during reward consumption.
Figure 3: Anatomically Distinct Subdivisions of pnVTAPnoc Neurons are Engaged During Reward Anticipation and Consumption.
(A) Cartoon schematic of fiber photometry setup.
(B) Summary of mean neural activity across various time intervals (intervals specified in Figure S3B) associated with reward anticipation and consumption during Pavlovian conditioning (n = 10 mice, 30 sessions) and operant task performance (n = 18 mice, 65 sessions; data presented as mean ± SEM; one sample t-tests: ***p<0.001).
(C) Number of licks per reward period during Pavlovian conditioning (n = 10 mice, 30 sessions) and operant task performance (n = 18 mice, 65 sessions; data presented as mean ± SEM).
(D) Heatmap of neural activity averaged across every reward period during FR3 (top panel) and PR (bottom panel) tasks. Each row corresponds to an individual mouse with anterior VTA fiber placement (n = 9 mice, 17 FR3 sessions, 17 PR sessions).
(E) Trace of neural activity averaged across every reward period during FR3 (top panel) and PR (bottom panel) tasks. Generated from all mice with anterior VTA fiber placement (n = 9 mice, 17 FR3 sessions, 17 PR sessions).
(F) Coronal section showing anatomical location of 400μm optic fiber implant placement for mice with anterior VTA fiber placements.
(G) Heatmap of neural activity averaged across every reward period during FR3 (top panel) and PR (bottom panel) tasks. Each row corresponds to an individual mouse with posterior VTA fiber placement (n = 9 mice, 16 FR3 sessions, 15 PR sessions).
(H) Trace of neural activity averaged across every reward period during FR3 (top panel) and PR (bottom panel) tasks. Generated from all mice with posterior VTA fiber placement (n = 9 mice, 16 FR3 sessions, 15 PR sessions).
(I) Coronal section showing anatomical location of 400μm optic fiber implant placement for mice with posterior VTA fiber placements.
(J) Pavlovian conditioning schedule and cartoon depicting time course of house light (CS) and sipper access (US) during Pavlovian conditioning.
(K) Trace of neural activity averaged across every reward period during Pavlovian conditioning. Generated from Pnoc-Cre mice with anterior VTA fiber placement (middle panel) (n = 5 mice, 15 sessions) and posterior fiber placement (bottom panel) (n = 5 mice, 15 sessions). Trace of neural activity also shown for DAT-cre mice (top panel) (n = 7 mice, 14 sessions) for comparison of DA cell and Pnoc cell activity over identical Pavlovian conditioning paradigm.
(L) Proportion of time spent near sipper (ROI) during reward omission periods. Data point left of dashed line represents the last rewarded trial on day 6 of Pavlovian conditioning (n = 10 mice, 10 sessions).
(M) Trace of neural activity averaged across every reward omission period during day 6 of Pavlovian conditioning. Mice with anterior VTA fiber placement (top panel) (n = 5 mice, 5 sessions) and posterior fiber placement (bottom panel) (n = 5 mice, 5 sessions).
Further, VTAPnoc activity was also recorded in a Pavlovian conditioning paradigm (Figure 3J). The cue was presented 6 seconds before the sipper to allow for dissociation of Pnoc+ neural activity during reward anticipation from neural activity during reward consumption. A parallel group of DAT-Cre mice (n=7) were also recorded from in an identical Pavlovian paradigm to allow for direct comparison of DA cell activity and Pnoc+ cell activity within the VTA in this task. Anterior pnVTAPnoc cells increased their activity during reward consumption while posterior VTAPnoc inputs decreased their activity during reward consumption (Figure 3K). Additionally, anterior pnVTAPnoc cells had decreased activity in response to a reward-predictive cue while posterior VTAPnoc inputs showed no significant change from baseline during the cue period. Anterior pnVTAPnoc cells also had sharply increased activity after the end of the 20 second reward period for Pavlovian and operant sessions (Figure 3E and 3K). Reward omission trials were also tested with CS presentation, but no US access (Figure 3J). The consumption-related activity of anterior and posterior VTAPnoc inputs was absent during the omission trials (Figure 3M) despite mice remaining near the sipper port during extinction (Figure 3L). Lick rate during reward was lowest during Pavlovian conditioning, higher for the FR3 task, and highest for the PR task (Figure 3C). For both anatomically and functionally distinct Pnoc+ VTA inputs, the intensity of fluorescence during reward corresponds to lick rate (Pearson’s correlation: r = 0.849, p* = 0.033) and is negligible when mice are not consuming reward during omission trials (Figure 3B). Although anterior pnVTAPnoc cells decrease activity during reward anticipation, they did not exhibit any signal that parallels reward prediction error signals associated with reward omission (Schultz et al., 1997, 2007; Hart et al., 2014). However, these neurons are indeed recruited when access to sucrose reward is taken away during the post-reward period (Figure 3B). Altogether, these findings suggest that pnVTAPnoc cells act to limit seeking behavior, but do not likely represent error in reward anticipation.
pnVTAPnoc Neurons are Molecularly Heterogeneous
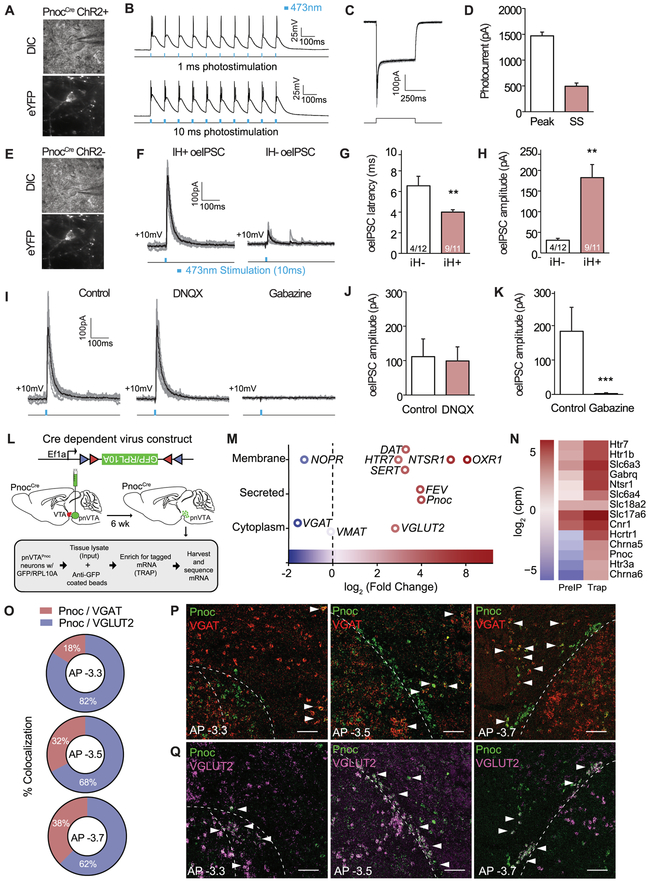
To determine whether pnVTAPnoc neuron form monosynaptic inputs onto VTA DA neurons, we performed whole-cell electrophysiology of PnocChR2 neurons within the VTA (Figure 4A, 4E). We found near 100% spike fidelity for all ChR2-eYFP expressing neurons at frequencies of 1, 5, 10, 20, and 40Hz (Figure 4B). (Figure 4C-D) In these neurons optical stimulation evoked robust inhibitory postsynaptic currents (oeIPSCs) in putative dopaminergic cells (IH+) and putative non-dopaminergic (IH−) neurons (Neuhoff et al., 2002). oeIPSCs latency was faster, as well as larger, in IH+ neurons when compared to IH− neurons (Figure 4G-H). We determined that these oeIPSCs inputs to be GABAergic as the oeIPSCs were abolished by blockade of GABA receptors via Gabazine, while unaffected by blockade of glutamate receptors via DNQX (Figure 4I-K). Although, these data suggest a role for GABA within the VTAPnoc neuron population, anatomical and photometry data warranted further investigation of their molecular and anatomical identity. Specifically, we conducted a transcriptomic survey of pnVTAPnoc neuron gene expression using Translating Ribosome Affinity Purification (TRAP) (Sakers, et al, 2017; Mulvey et al., 2018). We injected a Cre-dependent TRAP AAV into the pnVTA of Pnoc-Cre mice to selectively tag (Figure S4D) and isolate ribosome-bound mRNA from Pnoc+ neurons and performed TRAP followed by RNAseq (Figure 4L-N). We validated TRAP isolation with robust enrichment of Pnoc as well as a host of other GPCR and neuropeptide mRNAs in these neurons (see Figure 4N, S4N, Table S2). Notably, we detected expression of both vGLUT2 (vesicular glutamate transporter) and vGAT (vesicular GABA transporter), indicating presence of pnVTAPnoc neurons containing both glutamate and/or GABA. FISH for Pnoc+, vGAT, and vGLUT2 revealed that anterior pnVTAPnoc cells predominantly co-express vGLUT2 and the relative prevalence of Pnoc/vGAT coexpression increased toward posterior VTA expressing Pnoc neurons (Figure 4J-L, S4A-B), demonstrating that there are two distinct, overlapping Pnoc+ subpopulations. Pnoc colocalization with either vGAT or vGLUT2 appears to form “pockets” where pnVTAPnoc cells are strictly glutamatergic while PBP Pnoc cells are GABAergic. Furthermore, injection of AAV5-EF1α-DIO-ChR2-eYFP injection into vGAT-Cre or vGLUT2-Cre revealed distinct expression patterns as compared to the pnVTAPnoc cells (Figure S4E-F). Together, these data indicate that pnVTAPnoc neurons have GABAergic connectivity with DA cells, but these pnVTAPnoc neurons are also heterogeneous in anatomical location and transcriptomic identity.
Figure 4: pnVTAPnoc Neurons Synapse onto DA Neurons and Molecularly Heterogeneous.
(A) Differential interference contrast (DIC; top) and fluorescent (eYFP; bottom) images of patch recordings for Pnoc-CreChR2cells.
(B) Representative traces of slice electrophysiological recordings of pnVTA Pnoc-CreChR2cells following 1 and 10ms, 10Hz photostimulation.
(C) Representative traces showing photocurrent in ChR2-eYFP expressing neurons.
(D) Peak and steady-state photocurrent amplitudes in ChR2-eYFP expressing neurons (n=4).
(E) Representative images showing patch-clamp recordings from non-eYFP expressing VTA neurons.
F) Representative traces showing optically evoked inhibitory postsynaptic currents (oeIPSC) in IH+ (putative DA cells; n=11) and IH− (putative non-DA cells; n=12) neurons.
G) oeIPSC latency was faster in IH+ versus IH− neurons (p<0.05).
H) oeIPSC amplitude was larger in IH+ versus IH− neurons (p<0.05).
I) Representative traces showing oeIPSCs from VTA neurons before and after drug application.
J) oeIPSCs were unaffected by blockade of glutamate receptors.
K) oeIPSCs were abolished by blockade of GABA receptors.
(L) Cartoon depicting viral injection and TRAP of pnVTAPnoc neurons.
(M) Single-cell RNA-sequencing of TRAPed pnVTAPnoc neurons showing significantly expressed genes from membrane, secreted, and cytoplasm of Pnoc-labeled neurons (n=5, 5 slices per n).
(N) Heatmap from single-cell RNA-sequencing comparing Input and TRAP
(O) Pie charts depicting Pnoc / VGAT (blue) and Pnoc / VGlut2 percent co-expression as a function of anterior to posterior (AP) progression (n=4, 4 slices per n).
(P) Coronal images images of Pnoc (green) and VGAT (red) mRNA expression within the pnVTA as a progression from anterior to posterior (AP). Open arrows: no colabeling and filled-in arrows: colabeling.
(Q) Coronal images of Pnoc (green) and VGlut2 (purple) mRNA expression within the pnVTA from anterior to posterior (AP). Open arrows represent no co-labeling, and filled arrows represent colabeling.
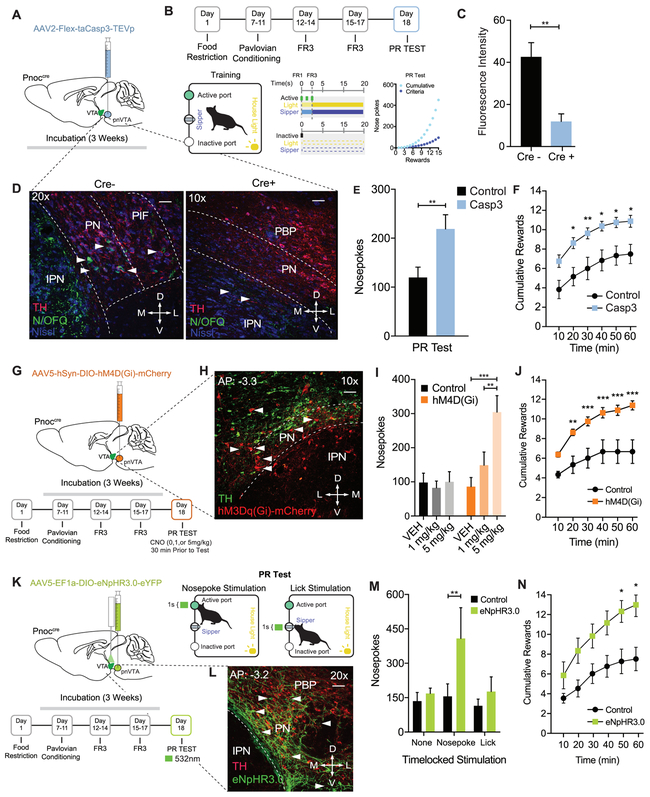
Selective Ablation and Inhibition of pnVTAPnoc Neurons Enhances Reward Seeking Breakpoint
To determine the functional necessity of the pnVTAPnoc neurons in regulating natural reward-seeking behavior, we utilized three separate, but parallel approaches to assess if deletion or inhibition of pnVTAPnoc neurons impacts motivation for rewards. First, we injected an AAV5-FLEX-taCasp3-TEVp into the pnVTA of Pnoc-Cre+ and Pnoc-Cre− mice to induce apoptosis in Cre-positive pnVTAPnoc neurons. This caused a significant loss in pnVTAPnoc neurons of Pnoc+ mice (Figure 5C-D, S5A). We observed that pnVTAPnoc neuron ablation increased responding under the PR task showing increases in total nosepokes, rewards received, as well as robust increases in operant responding across the entire PR test (Figure 5B,E-F, S5B-E). These manipulations increased breakpoint and seeking for reward (FR3) but did not impact reward consumption (i.e. lick rate) (Figure S5F). Taken together, these data demonstrate that selective removal of pnVTAPnoc neurons enhances PR responding for sucrose, implicating a necessary role for these unique pnVTA neurons in limiting motivation for rewards.
Figure 5: Selective Ablation and Inhibition of pnVTAPnoc Neurons Enhances Operant Responding for Natural Rewards.
(A) Cartoon depicting injection of AAV2-FLEX-taCasp3-TEVp into the pnVTA of Pnoc-Cre+ and Pnoc-Cre− mice.
(B) Schematic and timeline for the operant training schedule, FR, and PR test days.
(C) Quantification of fluorescence intensity from immunohistochemistry analysis for N/OFQ fluorescence in Pnoc-Cre+ and Pnoc-Cre− mice following cell ablation).
(D) Representative coronal images of the VTA and IPN showing immunohistochemistry for nociceptin (N/OFQ) and tyrosine hydroxylase (TH) staining in Pnoc-Cre− (left panel) and Pnoc-Cre+ (right panel) mice. Images show N/OFQ (green), DAPI (blue) and TH (red). Scale bars are 50 and 100μm, respectively.
(E) Nosepokes performed during PR test. Pnoc-CretaCasp3 mice have significantly increased nosepokes and rewards, compared to controls (n = 11 to 13: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for control versus Casp3 during PR test **p < 0.01,.
(F) Cumulative rewards received in Control and Pnoc-CretaCasp3 mice. Pnoc-CretaCasp3 mice show significantly increased number of rewards received, compared to controls (n = 11 to 13: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Control versus Casp3 during PR test *p < 0.05, **p<0.01.
(G) Calendar for injection of AAV5-hSyn-DIO-hM4D(Gi)-mCherry into the pnVTA of Pnoc-Cre+ and Pnoc-Cre− mice. Schematic and timeline for the operant training schedule and FR and PR test days.
(H) Coronal images of the pnVTA and IPN showing immunofluorescence staining tyrosine hydroxylase (TH) and hM4D(Gi)-mCherry following viral injection in Pnoc-Cre+ mice. Images show TH (green) and hM4D(Gi)-mCherry (red).
(I) Number of nosepokes performed during PR test. Pnoc-CrehM4D(Gi) mice show significantly increased active nosepokes compared to Controls (n = 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Vehicle versus 5mg/kg, ***p < 0.001, 1mg/kg versus 5mg/kg **p < 0.01.
(J) Cumulative rewards received in Control and Pnoc-CrehM4D(Gi) mice. Pnoc-CrehM4D(Gi) mice receive significantly more rewards over session, compared to controls (n = 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for control versus hM4D(Gi) during PR test, **p < 0.01, ***p < 0.001.
(K) Calendar for injection of AAV5- EF1α-DIO-eNpHR3.0-eYFP into the pnVTA of Pnoc-Cre+ and Pnoc-Cre− mice. Schematic and timeline for the operant training schedule, FR and PR test days.
(L) Coronal images of pnVTA immunofluorescence staining of tyrosine hydroxylase (TH) and eNpHR3.0-eYFP following viral injection in Pnoc-Cre+ mice. Images show TH (red) and eNpHR3.0-eYFP (green).
(M) Number of nosepokes performed during PR test. Pnoc-CreeNpHR3.0 mice show significantly increased active nosepokes compared to Controls following nosepoke-paired inhibition (n = 6: two-way repeated-measures ANOVA, Bonferroni post hoc; **p<0.01)
(N) Cumulative rewards received in Control and Pnoc-CreeNpHR3.0 mice. Pnoc-CreeNpHR3.0 mice receive significantly more rewards over session compared to control mice following nosepoke-paired inhibition (n = 6: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepoke-paired inhibition Rewards for Control versus Pnoc-CreeNpHR3 during PR test, *p<0.05.
We injected virus encoding the inhibitory DREADD receptor (AAV5-hSyn-DIO-hM4Di-mCherry into the pnVTA of Pnoc-Cre+ and Pnoc-Cre− mice (Figure 5G and 5H) and tested mice in a PR task following the administration of two CNO doses: 1mg/kg (i.p.) and 5mg/kg (i.p.) (Figure 5G). We found that chemogenetic inhibition of pnVTAPnoc neurons significantly increased nosepoke responding following CNO (5 mg/kg) and increased the number rewards received following both CNO doses (1 and 5 mg/kg) (Figure 5I, 5H, S5G and S5H). However, the above approaches do not assess how inhibiting pnVTAPnoc neurons during specific epochs of the behavioral task might modulate the motivation to obtain natural rewards. Therefore, in a parallel series of experiments, we injected an AAV5-EF1α-DIO-eNpHR3.0-eYFP, and chronically implanted bilateral fiber optics into their terminal region of the lateral VTA of Pnoc-Cre+ and Pnoc-Cre− mice (Figure 5K-L). We then assessed whether motivation to obtain sucrose rewards was altered by time-locked photoinhibition of pnVTAPnoc neurons during nosepoking or licking. Photoinhibition of pnVTAPnoc neurons was found to increase the total number of nosepokes and the number of total rewards during the PR task, but only when inhibition was paired to nosepoking behavior (Figure 5M-N, S5I-J). These data suggest that pnVTAPnoc neurons are necessary for operant responding for rewards, but not for consumption of a natural reward. Taken together, these data demonstrate that selective removal and inhibition of pnVTAPnoc neurons robustly enhances PR responding, implicating a key role for these neurons in regulating low-yield reward-seeking behavior.
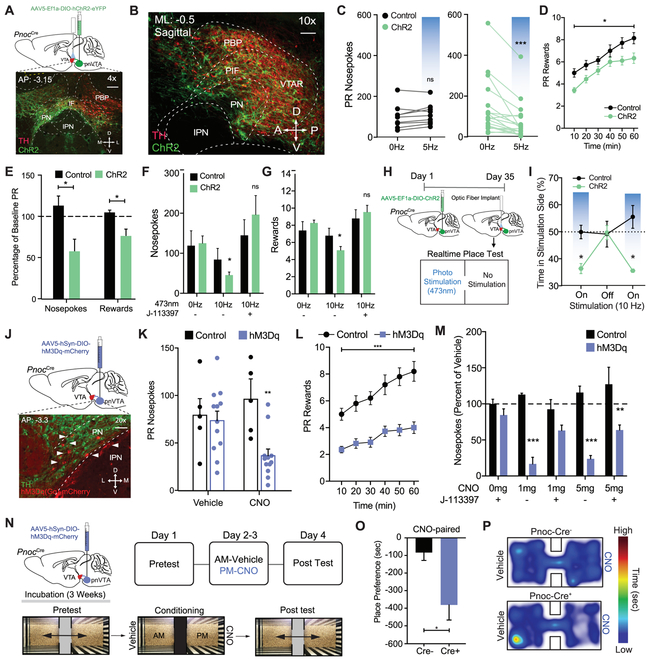
Optogenetic and Chemogenetic Stimulation of pnVTAPnoc Neurons Decreases Effort to Receive a Natural Reward and Promotes Aversion
To determine the sufficiency of pnVTAPnoc neurons in modulating sucrose reward seeking, we injected AAV5-EF1α-DIO-ChR2-eYFP, and chronically implanted a fiber optic into their terminal region of the lateral VTA of Pnoc-Cre+ and Pnoc-Cre− mice (Figure 6_A-B, S1A-B). During the PR task, mice received 60 minutes of 5Hz photo-stimulation or had the photo-stimulation time-locked (10Hz, 1 sec) to either their nosepoking or licking. We found that 5Hz stimulation of Pnoc+ VTA neurons and their terminals significantly decreased total nosepokes and rewards during FR3 and PR tests (Figure 6C-F, Figure S6E-F). We also determined whether the selective NOPR antagonist, J-113397, (3mg/kg, i.p.) would block this reduction in behavior. Indeed, antagonist administration prevented the photo-stimulated pnVTAPnoc-mediated suppression of sucrose seeking (Figure 6G), suggesting that tonic stimulation of pnVTAPnoc neurons releases NOPR agonist nociceptin. Time-locked stimulation did not affect sucrose seeking behaviors (Figure S6C), yet we found that nosepoke-paired stimulation led to either diminished (n = 7) or enhanced (n = 4) sucrose seeking (Figure S6D). These data suggest that while continuous, tonic unpaired pnVTAPnoc stimulation reduces PR performance, behavior-paired acute stimulation results in a divergent responding during the task, underscoring the additional complex features of this unique population within the pnVTA.
Figure 6: Optogenetic and Chemogenetic Stimulation of pnVTAPnoc Neurons Decreases Effort to Receive a Natural Reward and Promotes Aversion.
(A) Sagittal brain cartoon (top) of viral injection of ChR2 into the pnVTA of Pnoc-Cre+ mice and representative sagittal image (bottom) showing immunohistochemistry for ChR2 (green) and tyrosine hydroxylase (TH) (red).
(B) Calendar outlining timeline and stimulation parameters for operant training schedule during FR1, FR3, and PR tasks.
(C) Number of nosepokes during PR test. Pnoc-CreChR2 mice decrease responding during continuous 5 Hz (10ms) blue light stimulation compared to controls (n=12: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for 0Hz versus 5Hz during PR test, ***p<0.001).
(D) Number of rewards received over time during PR test. Pnoc-CreChR2 mice show a significant reduction in the number of rewards received during the PR test received following 5Hz stimulation compared to controls (n=12: Student’s t-test, *p<0.05)
(E) Percentage of baseline nosepokes and rewards during PR test. Pnoc-CreChR2 mice show a significant decrease in active nosepokes and number of rewards received during 5 Hz stimulation compared to baseline and controls (n=12: One way ANOVA, Bonferroni post hoc; Nosepokes for baseline versus 5Hz during PR test *p<0.05, Rewards for baseline versus 5Hz during PR test *p<0.05).
(F) Number of nosepokes and (G) rewards during PR test in Pnoc-CreChR2 mice that received photostimulation (10Hz, 10ms) and the NOPR antagonist (J-113397, 3 mg/kg). Pnoc-CreChR2 mice show a significant decrease in active nosepokes and rewards compared to controls during photostimulation that was rescued by treatment with J-113397 (n=12: two-way repeated-measures ANOVA, Bonferroni post hoc; Pnoc-CreChR2 0Hz versus 10 Hz, *p<0.05, 0Hz versus 10 Hz + J-113397, ns)
(H) Calendar outlining timeline for viral injection of ChR2 and VTA fiber optic implantation, followed by real time place testing.
(I) Time-lock (ON-OFF-ON) patterns of photostimulation-induced avoidance. Percentage of total time spent receiving 10Hz stimulation in control and Pnoc-CreChR2 mice (n=9 to 12: two-way repeated-measures ANOVA, Bonferroni post hoc; percentage of time spent in stimulation side for control versus Pnoc-CreChR2 at 10Hz *p < 0.05).
(J) Sagittal brain cartoon (top panel) of viral injection of hM3D(Gq)-mCherry into the pnVTA of Pnoc-Cre+ mice and representative coronal image (bottom panel) showing immunohistochemistry for hM3D(Gq)-mCherry (red) and tyrosine hydroxylase (TH) (green).
(K) Number of nosepokes during PR test. Pnoc-CrehM3D(Gq) mice show a significant decrease in active nosepokes CNO (5mg/kg) administration compared to controls (data presented as mean ± SEM, n=5 to 12: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for baseline versus CNO (5mg/kg) during PR test **p<0.01) Control mice show no effect in nosepokes or number of rewards received following CNO (5mg/kg) administration.
(L) Number of rewards received over time during PR test. Pnoc-CrehM3D(Gq) mice show a significant reduction in the number of rewards received during PR test received following CNO (5mg/kg) administration compared to controls (n=5 to 12: Student’s t-test, ***p<0.001)
(M) Number of nosepokes (as a % of vehicle treatment) during PR test in Pnoc-CrehM3D(Gq) mice that received CNO (1 and 5 mg/kg) and J-113397 (3 mg/kg). CNO treated mice show a significant decrease in nosepokes compared to controls that was partially rescued by treatment with J-113397 (n=5 to 12: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for baseline versus CNO (5mg/kg) during PR test **p < 0.01, Rewards for baseline versus CNO (5mg/kg) during PR test **p < 0.01).
N) Timeline for pnVTA viral injection and detailed calendar outlining CPP experimental paradigm.
(O) Amount of time spent on CNO-treated side during conditioned place post-test between Pnoc-Cre− and Pnoc-Cre+ mice. Post test reveals Pnoc-CrehM3D(Gq) mice spend significantly less time in the side previously paired with CNO (5mg/kg). (n=5, 12 sessions: Student’s t-test, Tukeys post-hoc, ****p<0.01).
(P) Representative heat map of relative time spent in CPP chambers for a Pnoc-Cre− and Pnoc-Cre+ mouse during CPP post-test following 5 mg/kg CNO treatment.
We next determined whether selective stimulation of pnVTAPnoc neurons causes a particular valence response (Namburi et al., 2016). We injected AAV5-DIO-EF1α-ChR2-eYFP into the pnVTA of Pnoc-Cre+ or Pnoc-Cre− mice and used a real-time place test (RTPT) paradigm (Figure 6H) to determine whether mice prefer or avoid neutral contexts paired with various photostimulation frequencies (0-60hz) (Al-Hasani et al., 2015). We found that photostimulation of pnVTAPnoc neurons (5Hz and 10Hz) produced significant real-time avoidance behavior (Figure S6H-I). This real time avoidance behavior is reversible, conserved following multiple test days, as well as within test (ON-OFF-ON), as demonstrated in Figure 6I.
In a parallel series of experiments, we determined the effects of stimulating pnVTAPnoc neurons via excitatory (hM3Gq) chemogenetic manipulation. We injected AAV5-hSyn-DIO-hM3Dq-mCherry into the pnVTA region of Pnoc-Cre+ and Pnoc-Cre− mice (Figure 6J) and tested mice in the PR task following CNO administration (1 and 5mg/kg, i.p.) We found that chemogenetic stimulation (5mg/kg CNO) of pnVTAPnoc neurons decreased operant responding and the number of rewards received during the PR task (Figure 6K-L, Figure S6K-L). In fact, we also found that CNO administration dose-dependently (1mg/kg and 5mg/kg) decreased the total number of nosepokes and the total number of sucrose rewards received in Pnoc-CrehM3D(Gq) expressing mice (Figure 6M, Figure S6M). Both 1mg and 5mg CNO doses reduced operant responding and subsequent reward received only in Pnoc-CrehM3D(Gq) expressing mice (Figure 6G-6H). Nosepoke responses and rewards for Pnoc-CrehM3D(Gq) mice treated with CNO + the NOPR antagonist J-113397 returned to similar levels as compared to mice following vehicle administration (Figure 6M, Figure S6M). These results demonstrate a reversal of CNO-induced reduction in PR responding and further indicate that stimulating pnVTAPnoc neurons releases nociceptin to act on NOP receptors.
We next determined whether stimulation of pnVTAPnoc neurons integrates salient negative affective information with associated environmental cues. Here we injected AAV5-hSyn-DIO-hM3Dq-mCherry in Pnoc-Cre+ and Pnoc-Cre− mice and measured conditioned place preference (CPP) for the context paired with DREADD agonist CNO (Figure 6N). We observed that repeated administration of CNO (5 mg/kg, i.p.) in a paired context induced a robust conditioned place aversion (CPA) only in Pnoc-CrehM3D(Gq) mice (Figure 6O-P), without impacting locomotor activity, (Figure S6N). These data indicate that pnVTAPnoc neuron activation drives aversion in neutral environments and integrates negative valence with relevant contextual stimuli.
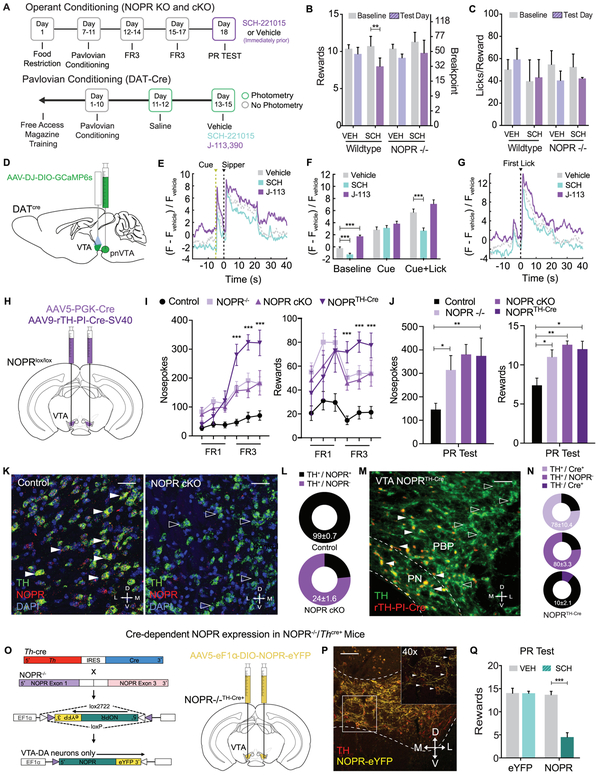
VTA NOPR Expression is Necessary and Sufficient for Constraining Reward Seeking
Our results above demonstrated involvement of both the neuropeptide nociceptin and NOPR in regulating sucrose seeking behavior; however, how and where these NOPR-mediated effects act is unknown. We next determined whether VTA NOPR expression is necessary and sufficient to coordinate this behavior. Using wildtype and NOPR knockout (KO) mice, we examined how selective NOPR agonist SCH-221510 administration impacts reward seeking within the PR task (Figure 7A). Here, we found that SCH-221510 administration (10mg/kg, i.p.) significantly reduced the number of nosepokes and rewards compared to baseline PR responding of wildtype mice, while having no significant effect in NOPR−/− mice (Figure 7B), complimenting previous experimental pnVTAPnoc manipulation results in Figure 6..Additionally, the administration NOPR agonist had no effect on the average lick rate during sucrose consumption (Figure 7C). This data indicates that exogenous NOPR stimulation is sufficient to reduce breakpoints for reward.
Figure 7: VTA Dopamine Neuron NOPR Expression is Necessary and Sufficient for Regulating Motivation for Sucrose.
(A) Calendar outlining timeline for operant training schedule in NOPR KO and cKO experiments or pavlovian training schedule for in vivo photometry recording of DAT-Cre experiments.
(B) Number of rewards and licks per reward during PR test following vehicle and SCH221510 (10mg/kg) administration. Wildtype (WT) mice show a significant decrease in the number of rewards received following SCH221510 (10mg/kg) administration compared to NOPR−/− mice (n=6 to 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Rewards for Baseline versus SCH221510 (10mg/kg) PR test **p < 0.01).
(C) Data depicting lick rate between vehicle and SCH221510 (10 mg/kg) treated WT and NOPR KO mice during PR testing. (n=6 to 8: two-way repeated-measures ANOVA, ns).
D) Cartoon representation of viral injection of GCaMP6s in the VTA of DAT-cre animals.
(E) Comparison of mean DA cell dynamics across reward-predictive cue and sipper presentation in Pavlovian conditioning paradigm with systemic activation or blockade of NOPR (n=7 animals, 7 sessions per drug treatment).
(F) Activity of DA cells during Pavlovian conditioning paradigm organized into 2-s time bins based on behavioral state of animal (n=7, 7 sessions per drug treatment; data represented as mean ± SEM; two sample t-tests: ***p<0.001).
(G) Comparison of mean DA cell dynamics across first lick event within reward period of Pavlovian conditioning paradigm with systemic activation or blockade of NOPR (n = 7 , 7 sessions per drug treatment).
(H) Cartoon depicting viral conditional knock out of NOPR in the VTA of NOPRloxP/YFP mice after bilateral injection of AAV5-PGK-Cre or AAV9-rTH-PI-Cre-SV40 into the VTA.
(I) Number of nosepokes and rewards during FR1 and FR3 test sessions between control, NOPR KO, NOPR cKO, and NOPRTH-Cre mice. NOPR KO, NOPR cKO, and NOPRTH-Cre mice show a significant increase in the number of rewards received and total nosepokes in comparison to WT mice (n=6 to 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes and Rewards for WT versus NOPR KO, NOPR cKO, and NOPRTH-Cre FR1 and FR3 tests ***p<0.001).
(J) Number of nosepokes and rewards during PR test sessions between control, NOPR KO, NOPR cKO, and NOPRTH-Cre mice. NOPR KO, NOPR cKO, and NOPRTH-Cre mice show a significant increase in the number of rewards received and total nosepokes in comparison to WT mice (n=6 to 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes and Rewards for WT versus NOPR KO and NOPR cKO PR tests, ***p<0.001).
(K) Representative 40X coronal images for in situ hybridization showing colabeling of NOPR (red) and TH (green) mRNA in NOPRlox/lox− (left panel) and NOPRlox/lox+ (right panel) mice that received injections of AAV5-PGK-Cre.
(L) Pie chart graph depicting %Co-expression of TH and NOPR mRNA in the VTA of NOPRlox/lox− (top panel) and NOPRlox/lox+ (bottom panel) mice that received injections of AAV5-PGK-Cre (n=4, 4 slices per n).
(M) Representative 20X coronal images for immunofluorescence showing colabeling of Cre (red) and TH (green) in NOPRlox/lox+ mice that received injections of AAV9-rTH-PI-Cre-SV40.
(L) Pie chart graph depicting %Co-expression of TH and Cre (top panel) and %Co-expression of TH and NOPR (bottom panel) in the VTA of NOPRlox/lox+ mice that received injections of AAV9-rTH-PI-Cre-SV40 (n=4, 4 slices per n).
(O) Cartoon for viral rescue of NOPR in VTA dopamine cells of NOPR−/− X TH-Cre mice.
(P) Representative image (10x) showing NOPR-eYFP infected VTA TH+-cells. Images show TH (red) and NOPR-eYFP (yellow). Inset image depicts 40x image. Arrows indicate TH and NOPR-eYFP co-labeling.
(Q) Number of rewards during PR test sessions following vehicle and SCH221510 (10mg/kg) administration. TH-Cre x NOPR−/− “rescue” mice show a significant decrease in the number of rewards received following SCH221510 (10mg/kg) administration compared to NOPR−/− mice (n=8 to 10: two-way repeated-measures ANOVA, Bonferroni post hoc; Training: Rewards for NOPR-rescue baseline PR test versus SCH221510 (10mg/kg) PR test ***p<0.001. No Training: Rewards for NOPR-rescue mice with Vehicle PR test versus SCH221510 (10mg/kg) PR test versus CNO (5mg/kg) during PR test ***p<0.001).
Next, to determine how and where NOPR mediates these effects on reward-seeking behavior, we recorded calcium transients in DA neurons in the VTA during pavlovian behavior following selective NOPR manipulation. In this experiment, DAT-Cre mice were injected with AAV-DJ-EF1α-DIO-GCaMP6s unilaterally into the VTA and implanted with optical fiber in the medial-lateral VTA (Figure 7D). Mice were trained in a Pavlovian conditioning task as described in Figure 3J. Mice were either injected with vehicle, SCH-221015 (10 mg/kg, i.p.), or J-113,397 (3 mg/kg, i.p.) prior to in vivo photometry recording of DA neuron activity during responding to cue and sucrose rewards (Figure 7A, S7C-E). NOPR activation significantly reduced overall baseline DA neural activity, while NOPR blockade increased baseline activity as compared to vehicle treated groups. This bidirectional effect was observed during consumption, but not during cue presentation (Figure 7E-G). Despite the effects on DA cell activity, there were no differences in lick rate or consumption of sucrose across treatments (Figure S7D). These data suggest that NOPR impacts DA neuron excitability during reward behavior.
To determine the necessity of NOPR expression specifically within the VTA on motivation and reward-seeking behavior, we further examined these behaviors following VTA-specific conditional deletion of NOPR. We first generated an original conditional NOPR knockout mouse (Figure S7A-B) in order to selectively remove NOPR from regions and cell-types of interest. Here, we bilaterally injected 350nl of AAV5-PGK-Cre or AAV9-rTH-PI-Cre-SV40 virus (for selective DA neuron expression) into the VTA of NOPRlox/lox (labeled NOPR cKO and NOPRTH-Cre, respectively) and control mice (Figure 7H). Using the same operant training and testing paradigms, we found that NOPR KO, VTANOPR cKO, and VTA NOPRTH-Cre cKO mice had significantly higher nosepokes and rewards received during operant responding as compared to littermate controls (Figure 7I). Similar to mice that have had pnVTAPnoc ablation or inhibition of pnVTA neurons (Figure 5), these NOPR KO, VTANOPR cKO, and VTA NOPRTH-Cre cKO mice showed a significant increase in responding for sucrose during the PR task (Figure 7J). Although, VTA NOPR cKO mice consumed more sucrose during the PR task, there was no difference in the average lick rate during reward consumption as a result of this manipulation (Figure S7F-G). Furthermore, to characterize this regional KO of NOPR, we used in situ hybridization for NOPR in VTA slices and found a significant reduction of NOPR mRNA expression in DA neurons (24±1.6%) in Cre-injected NOPRlox/lox mice, while leaving NOPR expression intact (99±0.7%) in control mice (Figure 7K-L). Additionally, VTA NOPRTH-Cre cKO mice show a significant reduction in NOPR expression as revealed by immunofluorescence of Cre in DA neurons (97±0.6%) and NOPR expression in DA neurons (20.3±3.3%) in rTH-PI-Cre-injected NOPRlox/lox mice (Figure 7M-N).
To determine the sufficiency of NOPR in VTA DA neurons to produce these effects on reward seeking, we generated a mouse line of constitutive NOPR KO mice crossed to a Th-Cre mouse line (Figure 7O). We then constructed an AAV5-EF1α-DIO-NOPR-eYFP virus and injected it into the VTA of NOPR−/− x TH-Cre+ mice to express NOPR only within VTA DA neurons (Figure 7O-P). We then trained these mice as in previous experiments and examined how the selective NOPR agonist SCH-221510 (10 mg/kg, i.p.) impacted PR responding for sucrose in mice that express NOPR only in VTA DA neurons. Here we found that SCH-221510 significantly reduced responding in NOPR−/−::NOPR-VTA mice, while leaving control (AAV5-EF1α-DIO-eYFP) animal responding was unaffected (Figure 7Q, S7I). This selective VTA DA NOPR expression and agonist stimulation did not alter lick rate or impact locomotor activity (Figure S7J-K). These results indicate a critical role for endogenous nociceptin and NOPR activity on DA neurons. Together these data strongly support the conclusion that VTA NOPR activation constrains motivation to seek rewards.
DISCUSSION
We generated a Pnoc-Cre mouse line to specifically target and isolate Pnoc-expressing inputs into the VTA and map endogenous sources of nociceptin. Following examination of Pnoc expression patterns in two new Pnoc reporter lines (Figure 1) we identified numerous brain regions rich in Pnoc-expressing neurons. Considering our data indicating monosynaptic inputs onto VTA DA neurons, we investigated multiple potential neurobiological sources of nociceptin inputs into the VTA, and found that the pnVTA not only expressed a distinct pattern of nociceptin neuron labeling, but was also comprised of likely dense inputs onto VTA DA neurons. Considering that the VTA and other ventral midbrain nuclei contain heterogeneous neuronal populations, no studies to date have demonstrated the function of this unique Pnoc-containing neuron population within the pnVTA region.
When examining the activity of pnVTAPnoc neurons during reward seeking conditions, we found they have relatively low activity when rewards are readily accessible and effort requirements are low during the PR task or during reward anticipation in Pavlovian conditioning tasks. These neurons are dynamically engaged during higher PR levels when mice are nosepoking infrequently for reward and not achieving sipper access (Figure 2G). In fact, we find that neural activity is highest when mice are reaching their breakpoint and terminate their reward-seeking behavior. Distinct activation of pnVTAPnoc neurons was also observed directly after the reward period when the sucrose sipper retracted and mice could no longer consume the reward. Although pnVTAPnoc neurons are very active when current reward access is removed, they surprisingly did not exhibit any significant signal associated with reward prediction errors that have been canonically observed in DA cells within the VTA (Figure 3B). This is a surprising finding as DA cells phasically increase firing in response to reward predictive cues in anticipation of reward and are also dramatically suppressed when expected rewards are not received (Schultz et al., 1997; Hart et al., 2014). Together, the endogenous activity of pnVTAPnoc neurons indicates that they do not signal errors in reward prediction, but rather signal to limit the motivation for reward. Indeed, mesolimbic DA is necessary for potentiating reward-seeking behavior when the effort required for reward is greater and the rate of reward attainment is lower, while DA activity is lower when future rewards are uncertain and the effort to reward payout ratio is greater (Gan, Walton, and Phillips, 2010; Hamid et al., 2016). Here we report that the pnVTAPnoc neurons are increasingly active as nosepoke rate declines and likely inhibit VTA DA neurons during low-yield reward seeking via release of nociception and subsequent NOPR activation. It is possible that pnVTAPnoc neurons increasingly suppress DA neuron tonic activity to diminish and regulate seeking for less attainable rewards.
Given endogenous pnVTAPnoc activity during reward seeking, we hypothesized that these neurons contribute inhibitory tone during sucrose seeking to limit motivation. Indeed, we observed increased PR performance following chronic ablation or inhibition of pnVTAPnoc neurons. Interestingly, PR performance also increased when inhibition of pnVTAPnoc neurons was time-locked only to nosepoke events (Figure 5K-5N). These data suggest that pnVTAPnoc neurons terminate seeking behavior particularly when the effort to obtain the reward increases. Accordingly, continuous pnVTAPnoc neuron stimulation reduced PR performance indicating that enhanced pnVTAPnoc activity is sufficient to diminish the pursuit of natural rewards, while NOPR antagonist administration reversed this effect. It was uncertain whether this loss in motivation is due to the promotion of a negative affective state or the enhancement of an existing inhibitory tone that coordinates the intersection of reward value and effort. pnVTAPnoc neuron stimulation resulted in active avoidance during real-time stimulation and mice avoided stimulation-paired environmental stimuli within the CPP assay. These data are indicative of a neuronal population that also promotes a negative affective state. Interestingly, this data parallels other studies that demonstrate that mice avoid VTA DA neuron inhibition (Tan et al., 2012; Danjo et al., 2014). pnVTA Pnoc neurons are likely positioned to receive input related to affective stimuli that subsequently drive inhibitory tone onto local VTA DA neuron populations, which would translate into reduced motivation in reward contexts and a negative affective state in non-reward contexts.
To further characterize this unique VTAPnoc population, we also assessed the activity of these neurons under a variety of reward epochs and behaviors. Examination of VTAPnoc activity during the reward access period and anatomical analysis of fiber optic placement location within the VTA revealed two additional discrete activity patterns during reward consumption; increased Pnoc neuron activity in the pnVTA and a suppression of activity in the posterior VTA. This bimodal population response may be due to the activation of a network of neurons that respond specifically to sucrose consumption. Indeed, it is known that posterior RMTG GABAergic inputs to the VTA are suppressed during reward consumption (Hong et al., 2011). It is possible that there are two Pnoc+ VTA input populations that are engaged during reward consumption: one posterior GABAergic pathway that is inhibited and one anterior glutamatergic pathway that is excited. In fact, our histological data show that the pnVTAPnoc neurons in anterior VTA predominantly express glutamatergic (Figure 4) markers. Recent work by Root et al. (2018) revealed that a majority VTA VGlut2-expressing neurons’ activity increases in response to aversive stimuli and decreases in response to rewarding stimuli. However, they also report other subpopulations that respond to different behaviorally relevant conditions. These are characteristics that we also observe within our cell population, as sucrose consumption enhances pnVTAPnoc neuron activity, while stimulation of pnVTAPnoc neurons drives aversion. Interestingly, our electrophysiological results revealed primarily GABAergic Pnoc neuron monosynaptic connectivity to putative DA neurons (Figure 4I-K). Although this establishes a subpopulation of GABA-containing Pnoc neurons within the VTA, the distinct pattern of expression we observed within the pnVTA was preserved throughout all viral infection experiments as described here and is indicative of an anterior, predominantly glutamatergic, population of pnVTAPnoc neurons. Specifically, we propose a distinct glutamatergic Pnoc population that is medial and ventral to DA neurons and a GABAergic Pnoc population that lies more lateral and dorsal to the VTA (Figure 4P-Q). In addition, TRAP RNAseq transcriptomic analysis revealed that pnVTAPnoc neurons are enriched in a number of unique receptors, transmitters, and peptides, including vGLUT2 (slc17a6), neurotensin-1 receptor (NTSR1), and orexin-1 receptor (HCRTR1) (Figure 4M-N). The enrichment of these specific genes within these neurons is of particular interest as these genes are also found within some VTA DA neurons (Baimel et al., 2015; Binder et al., 2001). Considering this data, pnVTAPnoc neurons may receive input from hypothalamic areas that help to coordinate reward motivation. This activity may provide feedback to VTA DA neurons to coordinate the intersection of reward value and reward-seeking action, however these hypotheses require future circuit-based experiments.
Recent studies have demonstrated that monoamine nuclei including the VTA, dorsal raphe, and locus coeruleus have a diverse host of neuronal populations based on their anatomical localization and projection target (Matthews et al., 2016; Seo et al, 2016; Beier et al, 2015). However, prior studies (Morales and Margolis, 2017) have yet to determine the connectivity and/or function of critical neuropeptide producing neurons in the VTA. Further investigation of VTA neuropeptide and neurotransmitter heterogeneity using intersectional genetic methods will elucidate how the VTA coordinates complex inputs to shape motivated behavior.
Given the concomitant intersection of results that indicate a unique pnVTAPnoc population regulates reward seeking by nociceptin release, we further determined whether there is a critical, necessary role for NOPR within the VTA in maintaining normal reward seeking. Global NOPR KO and conditional NOPR deletion from VTA DA cells dramatically enhanced reward-seeking behaviors in a manner similar to pnVTAPnoc neuron ablation and inhibition (Figure 5). This data indicates that, along with our electrophysiological and tracing studies, the action of pnVTAPnoc neurons on reward seeking is mediated through NOPR activation on dopaminergic VTA neurons. Interestingly, fiber photometry recordings of VTA DA activity during Pavlovian conditioning revealed that agonist activation of NOPR suppressed baseline DA neural activity. Phasic DA activity in response to the reward-predictive cue was left largely intact during NOPR activation while persistent DA activity during reward consumption was suppressed. This suggests that VTA NOPR activation may selectively limit PR performance by dramatically shifting dopaminergic tone. This finding is further supported by pnVTAPnoc manipulation affecting effort for reward, but not the rate of reward consumption. Lastly, we demonstrated that the conditional rescue and stimulation of NOPR specifically in VTA DA neurons greatly diminishes breakpoint responding. These data demonstrate the effectiveness of NOPR stimulation in inhibiting seeking for sucrose when it is exclusively expressed and stimulated in VTA DA neurons. Collectively, these data support a unique role of intra-VTA nociceptin release and subsequent NOPR activation acting to dynamically constrain DA neuron activity during reward-seeking behavior. In our case, an absent VTA nociceptin or NOPR system allows higher breakpoints for obtaining rewards in which a normally functioning NOPR system would typically engage and regulate appropriate reward seeking under various environmental conditions through DA neuron inhibition.
Our findings present a previously unknown pnVTA cell population of nociceptin-containing neurons that are positioned to suppress reward seeking via localized peptidergic action on NOPR-expressing VTA dopamine neurons. Understanding how this discrete pnVTA nucleus is critical to the regulation of motivation provides essential insights into the diverse behavioral functions of the VTA. How local VTA regions and neuropeptides are functionally defined at the circuit, cellular, and molecular level reveals insights into how the VTA coordinates homeostatic motivational balance during dysregulated motivational states like depression and addiction.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Additional information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michael Bruchas (mbruchas@uw.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Adult (20–35g) male and female prepronociceptin-IRES-Cre (Pnoc-Cre), NOPR KO, NOPR cKO mice were group housed, given access to food pellets and water ad libitum, and maintained on a 12 hr: 12 hr light:dark cycle (lights on at 7:00 AM). All mice were kept in a sound-attenuated, isolated holding facility in the lab one week prior to surgery, post-surgery, and throughout the duration of the behavioral assays to minimize stress. For ablation, chemogenetic, and optogenetic experiments, we used Cre- cage and littermate controls. For NOPR KO and NOPR cKO behavioral experiments, experimental mice were compared to age-matched wildtype controls. Unless otherwise noted, animals had ad libitum access to food and water. The mice were bred at Washington University in Saint Louis by crossing the Pnoc-Cre mice, NOPR KO, and NOPR cKO with C57BL/6 wild-type mice and backcrossed for seven generations. Additionally, where needed, Pnoc-Cre mice were then crossed to Ai9-tdTomato and Ai32-ChR2-eYFP mice on C57BL/6 background, bred, and backcrossed for seven generations. For the generation of NOPRlox/lox mice, Exon 4 of NOPR was flanked on both sides by loxP sites. All animals were drug and test naive, individually assigned to specific experiments as described, and not involved with other experimental procedures. Student’s t-tests did not detect any statistically significant difference when comparing animals grouped by sex, therefore male and female mice were combined to complete final group sizes. All animals were monitored for health status daily and before experimentation for the entirety of the study. All procedures were approved by the Animal Care and Use Committee of Washington University in St. Louis and performed in accordance with the National Institute of Health’s guide for the care and use of laboratory animals.
Generation of Pnoc-IRES-Cre mouse line
129 BAC genomic clones containing the Pnoc genes was used to target a cassette containing the Cre recombinase gene preceded by an internal ribosomal entry sequence (IRES-Cre) downstream of the Pnoc stop codon, so as to have endogenous Pnoc genes drive Cre recombinase expression. The Pnoc-IRES-Cre targeting constructs were electroporated into W4 ES cells and screened using an FRT-flanked neo-cassette. Targeted clones were injected into blastocysts. Chimeras were obtained and bred for germline transmission of the Pnoc-IRES-Cre allele. Following germline transmission, Pnoc-IRES-cre mice were crossed to Rosa-FLPR mice to remove the neo cassette at the 3′end of the IRES-cre sequence.
METHOD DETAILS
Tissue processing
Unless otherwise stated, animals were transcardially perfused with 0.1 M phosphate-buffered saline (PBS) and then 40 mL 4% paraformaldehyde (PFA). Brains were dissected and post-fixed in 4% PFA overnight and then transferred to 30% sucrose solution for cryoprotection. Brains were sectioned at 30 μM on a microtome and stored in a 0.01M phosphate buffer at 4°C prior to immunohistochemistry and tracing experiments. For behavioral cohorts, viral expression and optical fiber placements were confirmed before inclusion in the presented datasets.
Immunohistochemistry
Immunohistochemistry was performed as previously described by (Al-Hasani et al., 2013; Kim et al., 2013; McCall et al., 2015). In brief, mice were intracardially perfused with 4% paraformaldehyde, and then brains were sectioned (30μm) and placed in 0.1 M PB until immunohistochemistry. Free-floating sections were washed in 0.1 M PBS for 3 × 10 minutes intervals. Sections were then placed in blocking buffer (0.5% Triton X-100 and 5% natural goat serum in 0.1 M PBS) for 1 hr at room temperature. After blocking buffer, sections were placed in primary antibody (chicken anti-tyrosine hydroxylase,1:2000, Aves Labs, Inc.; rabbit anti-nociceptin, 1:500, abcam) overnight at room temperature. After 3 × 10-minute 0.1 M PBS washes, sections were incubated in secondary antibody (AlexaFluor 488 goat anti-rabbit; AlexaFluor 594 or 633 goat anti-chicken, Life Technologies) for 2 hours at room temperature, followed by subsequent washes (3 X 10 minute in 0.1 M PBS). Later, sections were incubated in NeuroTrace (435/455 blue fluorescent Nissl stain, ThermoFisher Scientific) for 1 hour, followed by 3 × 10 minute 0.1 MPBS then 3 × 10-minute 0.1 M PB washes. After immunostaining, sections were mounted and coverslipped with Vectashield HardSet mounting medium (Vector Laboratories) and imaged on a Leica TCS SPE confocal microscope.
RNAscope Fluorescent In Situ Hybridization (FISH)
Following rapid decapitation of mice, brains were flash frozen in −50°C 2-methylbutane and stored at −80°C for further processing. Coronal sections containing the BNST, CeA, and VTA/IPN regions, corresponding to the injection plane used in the behavioral experiments, were cut at 20μM at −20°C and thaw-mounted onto Super Frost Plus slides (Fisher). Slides were stored at −80°C until further processing. FISH was performed a ccording to the RNAScope® 2.0 Fluorescent Multiplex Kit for Fresh Frozen Tissue (Advanced Cell Diagnostics, Inc.) as described by Wang et al., 2012. Slides containing the specified coronal brain sections were fixed in 4% paraformaldehyde, dehydrated, and pretreated with protease IV solution for 30 mins. Sections were then incubated for target probes for mouse Pnoc, (accession number NM_010932.2, probe region 325-1263), VGlut (slc17a6, accession number NM_080853.3, probe region 1986-2998), VGat (slc32a1, accession number NM_009508.2, probe region 894-2037), Cre (accession number KC845567.1, probe region 1058-2032), NOPR (accession number NM_011012.5, probe region 988-1937, TH (accession number NM_009377.1, probe region 483 – 1603), DAT (slc6a3, accession number NM_010020.3, probe region 1486-2525) and GFP (accession number AF275953.1, probe region 12-686) for 2 hrs. All target probes consisted of 20 ZZ oliogonucleotides and were obtained from Advanced Cell Diagnostics. Following probe hybridization, sections underwent a series of probe signal amplification steps (AMP1-4) including a final incubation of fluorescently labeled probes (Alexa 488, Atto 550, Atto 647), designed to target the specified channel (C1-C3 depending on assay) associated with the probes. Slides were counterstained with DAPI and coverslips were mounted with Vectashield Hard Set mounting medium (Vector Laboratories). Images were obtained on a Leica TCS SPE confocal microscope (Leica), and Application Suite Advanced Fluorescence (LAS X) and ImageJ/Fiji software were used for analyses. Images for Pnoc Cre+ and Pnoc Cre− mice for quantification of Pnoc / Cre coexpression were carried out under the same standards (i.e. intensity, threshold, exposure time for each slide). To analyze the images, each image was opened in ImageJ software using the “Color Threshold” function, selecting the default threshold method and RGB color space. Within the threshold window, the default the settings were kept consistent for each slide. After adjusting for fluorescence threshold, we counted the total pixels of the fluorescent signal, assuming that each pixel represents a single molecule of RNA as per RNAscope’s methodology. A positive cell consisted of an area within the radius of a DAPI nuclear staining that measured at least 10 total positive pixels and an integrated density of >5. Three separate slices from the BNST, CeA, and pnVTA were used for each animal and that total is reflected in the data (n = 4).
Stereotaxic viral / optical fiber implant surgeries
After the mice were acclimated to the holding facility for at least seven days, the mice were anaesthetized in an induction chamber (1-4% isoflurane) and placed into a stereotaxic frame (Kopf Instruments, Model 1900) where they were maintained at 1-2% isoflurane. Mice were then injected unilaterally or bilaterally, depending on experimental paradigm, using a blunt needle (86200, Hamilton Company) at a rate of 100 nL / min. The type of virus, injection volume, and stereotaxic coordinates for each experiment are listed in Supplementary Table 1. Mice were allowed to recover for five weeks prior to behavioral testing, permitting optimal expression of the virus. For optogenetic experiments, one week prior to behavioral testing, intracranial optic fiber implants were directed above the VTA (AP −3.15, ML ± 0.5, DV −4.25). Implants were secured using at least two bone screws and a dental cement headcap (Lang Dental).
Generation of TRAP virus
The TRAP construct (eGFP/Rpl10a), a generous gift from N. Heintz lab, was PCR cloned into a commercial Gateway compatible entry vector (pENTR-3C), sequenced to confirm coding sequence, then transferred into an AAV-EF1α-DIO-Gateway destination vector using LR-clonase (Invitrogen) and following the manufacture’s protocol (Heiman et al., 2008). AAV-EF1α-DIO-Gateway plasmid was previously generated by replacing Chr2-eYfp from AAV-EF1α-DIO-ChR2-eYFP with an ATTR flanked Gateway acceptor cassette. The resulting AAV-EF1α-DIO-TRAP Plasmid was maxiprepped, sequence confirmed, then packaged by UNC Vector Core using standard protocols. Cre dependence was confirmed anatomically in independent mice and TRAP construct showed cytoplasmic and nucleolar localization within cells consistent with incorporation into ribosomes.
TRAP RNAseq Assay
TRAP was carried out as described in Sakers et al., 2017. Briefly, a 1mm coronal slice was dissected 6mm caudal to the base of the olfactory bulbs. Five slices (across 5 mice) were pooled and homogenized in cold lysis buffer (10 mM HEPES [pH 7.4], 150 mM KCl, 5 mM MgCl2, 0.5 mM dithiothreitol, 100 μg/ml cycloheximide, protease inhibitors, and recombinant RNase inhibitors) using a Teflon-glass homogenizer and a power drill. Homogenates were spun at 2,000xg for 10 minutes, and the resulting supernatant was lysed with 30mM DHPC and 1% NP40 followed by incubation on ice for 15 minutes. The lysate was then cleared at 20,000xg for 20 minutes, and the resulting supernatant was applied to EGFP-coated magnetic beads. 10% of this input was kept for a ‘Pre-IP’ sample. Immunoprecipitations were carried out for 4 hours at 4C with constant rotation. Four 1mL washes were carried out with wash buffer (10 mM HEPES [pH 7.4], 350 mM KCl, 5 mM MgCl2, 1% NP-40, 0.5mM dithiothreitol, 100 μg/ml cycloheximide and recombinant RNase inhibitors). RNA extraction using Trizol LS was carried out followed by Zymo RNA clean and concentrator-5 kit, with DNAse treatment, according to manufacturer’s guidelines.
Library Prep and Sequencing
cDNA was made and amplified from 1ng of RNA using the Ovation RNA Amplification System V2 from NuGEN. cDNA was sheared to ~200bp using a Covaris sonicator. RNA-sequencing libraries were made from 600ng of sheared cDNA with the NEBNext Ultra DNA Library Prep Kit for Illumina. ~90-95M 50bp reads were obtained, per sample, on an Illumina HiSeq 2500 and analyzed using an in-house pipeline as described in Sakers et al., 2017. Briefly, reads were trimmed using Trimmomatic (v0.33), aligned to the genome using STAR (Mouse Ensembl 77) and counted with HTseq. Counts per million analyses were performed in R using the edgeR package (R Foundation for Statistical Computing, Vienna, Austria).
Gene ontology
Gene ontology analysis was performed using the BiNGO application from Cytoscape (v3.4.0). Enriched genes were identified with a Benjamini-Hochberg corrected Hypergeometric test, at a significance level of p < 0.05. Pre-IP genes with counts above 2 were used as a reference set for enrichment.
Patch-clamp electrophysiology
Mice were anesthetized with pentobarbital (50 mg/kg) before transcardial perfusion with ice-cold sucrose cutting solution containing the following (in mM): 225 sucrose, 119 NaCl, 1.0 NaH2P04, 4.9 MgCl2, 0.1 CaCl2, 26.2 NaHCO3, 1.25 glucose, 305 mOsm. Brains were then rapidly removed, and coronal sections 300μm thick were taken using a vibratome (Leica, VT 1200). Sections were then incubated in aCSF (32°C) containing the following (in mM): 119 NaCl, 2.5 KCl, 1.0 NaH2P04, 1.3 MgCl, 2.5 CaCl2, 26.2 NaHCO3, 15 glucose, 305 mOsm. After an hour of recovery, slices were constantly perfused with aCSF (32°C) and visualized using differential interference contrast through a 40x water-immersion objective mounted on an upright microscope (Olympus BX51WI). Whole-cell recordings were obtained using borosilicate pipettes (3–5 MΩ) back-filled with internal solution containing the following (in mM): 130 K gluconate, 10 KCl, 10 HEPES, 10 EGTA, 2 MgCl2, 2 ATP, 0.2 GTP (pH 7.35, 270-285 mOsm). Current-clamp recordings were obtained from eYFP-expressing neurons to identify spike fidelity in optogenetic stimulation. During recordings, a train of 10 action potentials were evoked through presentation of a blue LED (488nm, 1mW, 1 and 10ms pulses) at frequencies of 1, 5, 10, 20, and 60Hz. Spike fidelity was quantified as the percentage of light presentations that resulted in action potential induction. Data acquisition occurred at 10 kHz sampling rate through a MultiClamp 700B amplifier connected to a Digidata 1440A digitizer (Molecular Devices) and were analyzed using threshold analysis in Clampfit 10.3 (Molecular Devices).
Behavior
All behaviors were performed within a sound-attenuated room maintained at 23°C at least one week after habituation to the holding room and the final surgery. For all experiments, mice were brought into the experimental behavior space and allowed to acclimate for at least 30 minutes. For all experiments, experimenters were blinded to mouse genotype or experimental manipulation. For chemogenetic and optogenetic behavior experiments, CNO dose and frequency of light stimulation were randomly assigned using block randomization, unless otherwise indicated. All pharmacological interventions (e.g. agonists and antagonists) were randomized and counterbalanced via block randomization prior to experimentation. Replication of operant responding behavior (e.g. PR test) was only used to compare experimental and control treatments with treatments counterbalanced between all mice. Sample-size estimation was determined via power analysis (G*Power 3; Faul et al., 2007) with a power of 0.85 and a standard deviation of 15-20% of the mean. Lighting was stabilized at ~1,500 lux for aversion behaviors, ~250 lux for anxiety-like behaviors. Movements were video recorded via CCD camera and analyzed using Ethovision XT 11 (Noldus Information Technologies). At the end of each study, mice were perfused with 4% paraformaldehyde followed by anatomical analysis to confirm injection sites and cell-type-specific expression.
Operant Progressive-Ratio Test
Food restricted (~90% of free feeding body weight) mice were placed in a Med-Associates operant conditioning box and subjected to magazine training during which a retractable lickometer containing a 10% sucrose solution extended for 20s at 20 random intervals for 60 minutes. Each sipper presentation was accompanied by illumination of a houselight. Following magazine training, mice were trained to nosepoke for lickometer access on a fixed ratio 1 (FR1) for 3 days (60 minutes/session). This required the mouse to nosepoke in the active nosepoke port one time to receive 20 seconds of access to the sipper. Following FR1 training, the ratio was increased to an FR3 schedule requiring the mouse to perform 3 active port nosepokes to receive access to the sipper. This schedule was used for 3 days (60 minutes/session). Finally, mice were given a progressive ratio schedule (PR) task following the geometric progression, nj = 5e j/5− 5, in which the criteria for rewards increased in an exponential manner (1, 2, 4, 6, 9, 12…) over the training period (Richardson and Roberts, 1996). For photostimulation and photoinhibition, mice received 5 Hz or 10 Hz stimulation (473 nm, 10 ms pulse width, ~10 mW light power) and (532 nm, ~10 mW light power), respectively upon placement into the operant chamber. During all optogenetic experiments, mice were counterbalanced to receive the appropriate photostimulation or photoinhibition parameters. In photostimulation experiments, animals received 5Hz stimulation during the entire PR session, 10Hz stimulation time-locked to nosepoking during the PR session, or 10Hz stimulation time-locked to licking of sucrose reward during PR session. In photoinhibition experiments, animals received either 1 sec of illumination time-locked to nosepoking during the PR session or 1 sec of illumination time-locked to licking of sucrose reward during PR session. For chemogenetic experiments, mice received an injection of vehicle or CNO (1 mg/kg; 5 mg/kg, i.p.) 30 minutes prior to being placed in the operant chamber on PR test day. For antagonist experiments, NOPR antagonist, J-113397 was administered (3 mg/kg, i.p.) 15 minutes prior to behavioral test.
Pavlovian Conditioning Paradigm
One week prior to Pavlovian conditioning, mice were food restricted down to ~90% of free feeding body weight. A subset of the Pnoc-Cre fiber photometry mice (n = 10) and DAT-Cre fiber photometry mice (n = 7) were trained to associate illumination of a house light (CS) with access to a sipper with 10% sucrose solution (US) within a Med-Associates operant box (ENV-307A). The house light would illuminate 6 s prior to sipper presentation. The sipper remained accessible for 20 seconds before it retracted and the house light shut off. A randomized intertrial interval of between 30-90 seconds separated consecutive trials. Pavlovian conditioning sessions lasted for 30 minutes, over which an average of 19-20 rewards were presented. For reward omission sessions, the first 10 rewards were cued and subsequently delivered as normal and every following trial only involved house light illumination with no sipper presentation. Simultaneous fiber photometry recordings were made during Pavlovian conditioning sessions as indicated in Results section. NOPR antagonist, J-113397 (3 mg/kg, i.p.) or SCH-221510 (10mg/kg, i.p.) were administered 15 minutes prior to Pavlovian conditioning.
In Vivo Fiber Photometry
Fiber photometry recordings were made throughout the entirety of 30-minute Pavlovian conditioning sessions and 1-hour FR3 and PR sessions. Prior to recording during Pavlovian or operant behavior sessions, an optic fiber was attached to the implanted fiber using a ferrule sleeve (Doric, ZR_2.5). Two LEDs were used to excite GCaMP6s. A 531-Hz sinusoidal LED light (Thorlabs, LED light: M470F3; LED driver: DC4104) was bandpass filtered (470 ± 20 nm, Doric, FMC4) to excite GCaMP6s and evoke Ca2+-dependent emission. A 211-Hz sinusoidal LED light (Thorlabs, LED light: M405FP1; LED driver: DC4104) was bandpass filtered (405 ± 10 nm, Doric, FMC4) to excite GCaMP6s and evoke Ca2+-independent isosbestic control emission. Prior to recording, a 120 second period of GCaMP6s excitation with 405 nm and 470 nm light was used to remove the majority of baseline drift. Laser intensity for the 470 nm and 405 nm wavelength bands were measured at the tip of the optic fiber and adjusted to ~50 μW before each day of recording. GCaMP6s fluorescence traveled through the same optic fiber before being bandpass filtered (525 ± 25 nm, Doric, FMC4), transduced by a femtowatt silicon photoreceiver (Newport, 2151) and recorded by a real-time processor (TDT, RZ5P). The envelopes of the 531-Hz and 211-Hz signals were extracted in real-time by the TDT program Synapse at a sampling rate of 1017.25Hz
Photometry Analysis
Custom MatLab scripts were developed for analyzing fiber photometry data in context of mouse behavior. The isosbestic 405 nm excitation control signal was subtracted from the 470 nm excitation signal to remove movement artifacts from intracellular Ca2+-dependent GCaMP6s fluorescence (see Figure S2A). Baseline drift was evident in the signal due to slow photobleaching artifacts, particularly during the first several minutes of each hour-long recording session. A double exponential curve was fit to the raw trace and subtracted (see Figure S2B) to correct for baseline drift. After baseline correction, the photometry trace was z-scored relative to the mean and standard deviation of the hour-long session. The post-processed fiber photometry signal was analyzed in the context of animal behavior during Pavlovian conditioning and operant task performance. Pearson correlations, one sample t-tests, two sample t-tests and two-way ANOVAs were performed using standard MATLAB functions “corr”, “ttest”, “ttest2” and “anovan”, respectively. ΔF/F was calculated as individual fluorescence intensity measurements relative to median fluorescence of entire session for 470nm channel.ΔF/F for DA cell NOPR manipulation experiments (see Figure 7) was calculated as individual fluorescence intensity measurements relative to median fluorescence of the entire vehicle treatment session. Recorded calcium dynamics were averaged into 2-second time bins and functionally categorized based on behavioral state of the animal: baseline activity during inter-trial interval, reward anticipation activity during cue presentation, or consumption activity when cue is present and animal is actively licking. A minority of scheduled FR3 and PR recording sessions were either excluded from analysis due to animal licking while sipper was retracted or not recorded due to fiber optic implant detachment before behavioral schedule was completed. 65/65 Pavlovian conditioning sessions, 33/36 planned FR3 sessions and 32/36 planned PR sessions were recorded and retained.
Real-Time Place Testing
We used custom-made unbiased, balanced two-compartment conditioning apparatus (52.5 × 25.5 × 25.5 cm) as described previously (Jennings et al., 2013; Stamatakis and Stuber, 2012). Each compartment was assigned either constant laser stimulation or no stimulation in a counterbalanced manner. Each animal was connected to a 473 nm laser via 200μm fiber optic patch cable then placed into the non-stimulation compartment at the onset of the experiment. Mice were allowed to freely roam between the two compartments for 30 minutes. Entry into the stimulation-paired compartment triggered constant photostimulation at either 5Hz, 10Hz, 20Hz or 60Hz (473 nm, 10 ms pulse width, ~10 mW light power) while the mouse remained in the stimulation-paired compartment. Entry into the non-stimulation compartment ended the photostimulation. The compartment paired with photostimulation was counterbalanced across all mice. Time spent in each compartment and total distance traveled for the entire 30-minute trial was recorder via CCD camera and measured using Ethovision 10 (Noldus Information Technologies, Leesburg, VA). Real-time preference was calculated by dividing the time spent in the stimulation-paired side by the total time in the apparatus.
Conditioned Place Preference
We used a modified three-chamber CPP apparatus consisting of two square boxes (27 cm × 27 cm) that served as the conditioning chambers separated by a small center area that served as the passageway (5 cm wide × 8 cm long) between boxes. Boxes had 2.5 cm black-and-white vertical stripes or horizontal stripes and floors were covered with 500 ml of bedding on each side. The floor of the center area was smooth Plexiglas. Mice were transported to the CPP behavior testing room and handled once per day for at least 7 days before behavioral testing. Mice were then conditioned using a semi-biased and counterbalanced CPP. On day one, mice were pretested for an initial bias to the conditioning apparatus and allowed to explore all three regions of the box drug-free for 20 minutes. Mice were paired in a counterbalanced fashion. Mice that spent >300 s on one side during the pretest were excluded due to potential side bias. Conditioning occurred over the following 2 days in which mice received a subcutaneous injection of saline in the morning and immediately confined to one side of the CPP box for 20 min. In the afternoon, at least 4 hours after the morning conditioning session, mice received an injection of CNO (5 mg/kg, i.p.) and were immediately confined to the opposite side for 20 min. This dose of CNO has been shown to be effective in activating DREADDS. Control (vehicle-injected) mice received saline injections during both A.M. and P.M. conditioning sessions. The following day, mice were tested for a preference for the CNO-paired side following the same procedure as the pretest. Preference scores were calculated by subtracting time spent in the CNO-paired side during the pretest from time spent in the CNO-paired side during the posttest.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses
All data collected were averaged and expressed as mean ± SEM. Statistical significance was taken as *p < 0.05, **p < 0.01, and ***p < 0.001, as determined by Pearson’s correlation, student’s t-test, one-way ANOVA or a two-way repeated-measures ANOVA followed by Bonferroni post hoc tests as appropriate. For in situ hybridization and electrophysiology data, we used Student’s t-test. For photometry experiments, we used Pearson’s correlation and student’s t-tests, as appropriate. For ablation, chemogenetic, and optogenetic behavioral experiments, we used one-way or two-way repeated-measures ANOVA followed by a Bonferroni post hoc tests, as described in Figures 4-6. For NOPR KO and NOPR cKO behavioral experiments, we used two-way repeated-measures ANOVA followed by a Bonferroni post hoc test, as described in Figure 7. All n values for each experimental group are described in the appropriate figure legend. For behavioral experiments, group size ranged from n = 5 to n = 18. For in situ hybridization quantification experiments, slices were collected from 4 mice, with data averaged from 3-4 slices per mouse (n = 4). For electrophysiology experiments, the number of cells recorded were as follows: n = 4 for recorded Pnoc-CreChR2 neurons, n = 11 for putative dopamine neurons, and n = 12 for putative non-dopamine neurons. For TRAP-RNA sequencing experiment, tissue slices were collected from 5 mice, with data averaged from 5 slices per mouse (n = 5). Statistical analyses were performed in GraphPad Prism 7.0 (Graphpad, La Jolla, CA) and MATLAB 9.1 (The MathWorks, Natick, MA).
DATA AND SOFTWARE AVAILABILITY
RNA sequencing data for pnVTAPnoc neurons from Figure 4 have been deposited and are available from GEO (Accession: GSE108813). Custom MATLAB analysis code was created to appropriately organize, process, and combine photometry recording data with associated behavioral data. Analysis code for photometry from Figure 2 and 3 is available online at: www.github.com/BruchasLab. The full behavioral data set supporting the current study are available from the corresponding author upon request.
Supplementary Material
Table S1. Related to STAR Methods Section: Stereotaxic viral / optical fiber implant surgeries. List of viruses used in stereotaxic surgery
Table S2. Related to Figure 4: pnVTAPnoc TRAP: Genes of Interest
Movie S1. Related to Figure 2: pnVTAPnoc Neural Activity During Breakpoint
Figure S1. Related to Figure 1: Anatomical Identification of Endogenous Pnoc-expressing VTA Inputs
(A) Schematic of targeting vector for generation of Pnoc-Cre mouse line.
(B) Representative southern blot of Pnoc-Cre knock-in
(C) Quantification of Cre expression within Pnoc-expressing cells in the BNST (1988/2122 cells), CeA (786/805 cells), and pnVTA (435/450 cells). (n=4, 4 mice, 3 slices each)
(D) Confirmation and validation of Pnoc-Cre mouse line depicting coronal images for Pnoc in Pnoc-Cre- mice for in situ hybridization colocalization of Pnoc (green) and Cre (red) for coronal images of BNST, CeA, and IPN/VTA regions.
(E) Confirmation of Pnoc-labeling within coronal images of the dentate gyrus (DG) of the hippocampus from left to right of: Pnoc mRNA labeling from mouse Allen Brain Atlas, RNAscope in situ hybridization of DAPI (blue) and Pnoc mRNA (green), Pnoc (red) labeling in Pnoc-Cre x Ai9-tdTomato against Nissl (blue) immunostaining, and Pnoc (green) labeling in Pnoc-Cre × Ai32-ChR2/eYFP against Nissl (blue).
(F) Confirmation of Pnoc-labeling within coronal images of the septum from left to right of: Pnoc mRNA labeling from mouse Allen Brain Atlas, RNAscope in situ hybridization of DAPI (blue) and Pnoc mRNA (green), Pnoc (red) labeling in Pnoc-Cre × Ai9-tdTomato against Nissl (blue) immunostaining, and Pnoc (green) labeling in Pnoc-Cre × Ai32-ChR2/eYFP against Nissl (blue).
(G) Coronal images depicting cre-dependent helper (red channel, left panel) and monosynaptic rabies (green channel, right panel) viral expression in the VTA / IPN regions of DAT-Cre mice corresponding to Figure 1F. PBP- parabrachial pigmented nucleus. Representative image from n=3.
(H) Coronal images that correspond to Figure 1H showing GFP (red, right panel) and Pnoc (green, left panel) mRNA following recombinant AAV helper viral and rabies viral injections into the VTA of DAT-Cre mice PN-paranigral, PBP- parabrachial pigmented nucleus. Representative images from n=3.
(I) Cartoon depicting recombinant retrograde AAV2 virus injection into the VTA of Pnoc-Cre mice. Coronal image depicts GFP (green) Pnoc+ cells in the paranigral (PN) region of the VTA against DAPI (blue) and tyrosine hydroxylase (red) immunostaining. PBP- parabrachial pigmented nucleus. Representative images taken from n=3.
(J) In situ hybridization colocalization of Pnoc (green) and DAT (red) for coronal images of zoomed VTA regions. Closed arrows represent colocalization of Pnoc and DAT, and empty arrows represent Pnoc labeled cells that are absent of DAT.
(K) Pie chart depicting quantification of Pnoc and DAT colocalization within the paranigral VTA (pnVTA) (n=3).
Figure S2. Related to Figure 2: Pnoc+ VTA Inputs are Engaged During Low-Yield Reward-Seeking
(A) Raw transduced photon emissions from excitation with 405 nm light and 470 nm light.
(B) Double exponential curve (red line) fit to signal after subtraction of 405 nm channel from 470 nm channel.
(C) Resulting baseline-corrected signal after subtraction of double exponential curve.
(D) Maximum activity (maximum 0.5 seconds of activity prior to reward epoch) for each FR3 level (n = 18 mice, 33 sessions: Pearson’s correlation, *p = 0.033, r = −0.073).
(E) Maximum activity (maximum 0.5 seconds of activity prior to reward epoch) relative to nosepoke criterion for each PR level (n = 18 mice, 32 sessions: Pearson’s correlation, ***p<0.001, r = 0.320).
(F) Mean neural activity during active nosepoking (see Figure 2H) relative to proportion of nosepokes until breakpoint for each PR session. Same data as Figure 2G, but split based on anatomical location of fiber implant within VTA (see Figure 3). (n = 18 mice, 32 sessions; Two-way repeated measures ANOVA: interaction, F = 0.49, p = 0.883).
Figure S3. Related to Figure 3: Anatomically Distinct Subdivisions of VTA Pnoc+ Neurons are Engaged Differentially During Reward Anticipation and Consumption
(A) Immunohistochemistry for GCaMP6s and tyrosine hydroxylase (TH) staining in Pnoc-Cre mice. Coronal sections show GCaMP6s (green), TH (red) and fiber implant tract (yellow) at various anterior/posterior coordinates throughout VTA.
(B) Time intervals relative to reward period that were used to calculate mean neural activity in Figure 3B. Trace of mean neural activity (red) taken from Figure 3K (middle panel) for reference.
(C) Immunohistochemistry for GCaMP6s and tyrosine hydroxylase (TH) staining in DAT-Cre mice. Coronal sections show GCaMP6s (green), TH (red) and fiber implant tract (yellow) at representative implant site.
(D) Coronal section cartoon showing anatomical location of 400μm optic fiber implant placement for DAT-Cre mice.
(E) Raster plot depicting reward consumption (lick rate) during a representative FR3 task session with simultaneous fiber photometry recording.
(F) Raster plot depicting reward consumption (lick rate) during a representative PR task session with simultaneous fiber photometry recording.
Figure S4. Related to Figure 4: pnVTAPnoc Neurons are molecularly heterogeneous
(A) Representative coronal images of DAPI (blue), Pnoc (green) and VGAT (red) mRNA expression within the pnVTA as a progression from anterior to posterior (AP). Open arrows represent no colabeling and filled-in arrows represent colabeling.
(B) Representative coronal images of DAPI (blue), Pnoc (green), VGlut2 (purple) mRNA expression within the pnVTA as a progression from anterior to posterior (AP). Open arrows represent no colabeling, and filled-in arrows represent colabeling.
(C) Scatterplot representing enriched mRNA (10 Fold) in pnVTAPnoc neurons. Pnoc presented in blue.
(D) Representative coronal image showing immunohistochemistry for AAV8-bacTRAP-eYFP and tyrosine hydroxylase (TH) staining. Images show bacTRAP (green), and TH (red).
(E) Representative VTA ChR2 expression patterns in vGlut2-Cre and vGAT-Cre mice.
Figure S5. Related to Figure 5; Selective Ablation and Inhibition of pnVTAPnoc Neurons Enhances Operant Responding for Natural Rewards
(A) Representative coronal images of the pnVTA and IPN showing in situ hybridization for nociceptin (N/OFQ) mRNA following viral injection in Pnoc-Cre− (left panel) and Pnoc-Cre+ (right panel) mice. Images show N/OFQ (green).
(B) Number of nosepokes (left panel) and rewards (right panel) across 3 days of FR1 and 3 days of FR3 sucrose self-administration. Pnoc-CretaCasp3 mice show significantly increased nosepokes and rewards received, compared to controls (n = 11 to 13: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Control versus Casp3 on day 2 and 3 of FR3 training *p < 0.05, Rewards for control versus Casp3 on day 2 and 3 of FR3 training *p < 0.05).
(C) Cumulative nosepokes across 60 min session Pnoc-Cre+ and Pnoc-Cre− mice that received injections of AAV2-FLEX-taCasp3-TEVp. (n = 11 to 13: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Control versus Casp3 **p < 0.05, **p < 0.01).
(D) Total nosepokes for each 10 min epoch across 60 min session in Pnoc-Cre+ and Pnoc-Cre− mice that received injections of AAV2-FLEX-taCasp3-TEVp. (n = 11 to 13: two-way repeated-measures ANOVA, ns).
(E) Average number of rewards received during PR test. Pnoc-CretaCasp3 mice show significantly increased number of rewards received, compared to controls (n = 11 to 13: unpaired t-test, Rewards for Control versus Casp3 during PR test *p < 0.05).
(F) Average number of licks per reward performed during PR test. Pnoc-CretaCasp3 mice mice show increased number of licks per reward received compared to Controls (n = 11 to 13: unpaired t-test, licks per reward for Control versus Casp3 during PR test, p = 0.08).
(G) Cumulative nosepokes across 60 min session of Pnoc-CrehM4D(Gi) given vehicle or CNO (5mg/kg). Pnoc-CrehM4D(Gi) mice show significantly increased number of cumulative nosepokes following CNO administration, compared to Vehicle. (n = 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Vehicle versus CNO, * p < 0.05, *** p < 0.001).
(H) Total nosepokes for each 10 min epoch across 60 min session of Pnoc-CrehM4D(Gi) mice given vehicle or CNO (5mg/kg). (n = 8: two-way repeated-measures ANOVA, ns).
(I) Average number of rewards received during PR test. Pnoc-CrehM4D(Gi) mice show significantly increased number of rewards received following CNO (5mg/kg administration), compared to vehicle treatment. (n = 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Rewards for Vehicle versus 5mg/kg, ***p < 0.001, 1mg/kg versus 5mg/kg **p < 0.01).
(J) Average number of licks per reward performed during PR test. There is no significant difference in lick rate between Control and Pnoc-CrehM4D(Gi) mice following administration of all doses of CNO (n=8: two-way repeated-measures ANOVA, ns).
(K) Cumulative nosepokes across 60 min session of Pnoc-CreeNpHR3.0 mice given either no inhibition or nosepoke-paired photoinhibition. Pnoc-CreeNplHR3.0 mice show significantly increased number of cumulative nosepokes following nosepoke-paired photoinhibition, compared to Baseline. (n=6: two-way repeated-measures ANOVA, Bonferroni post hoc; Cumulative nosepokes for Baseline versus Nosepoke, *p < 0.05, **p < 0.01).
(L) Total nosepokes for each 10 min epoch across 60 min session for Pnoc-CreeNpHR3.0 mice given either no inhibition or nosepoke-paired photoinhibition. (n = 6: two-way repeated-measures ANOVA, ns).
(M) Average number of rewards received during PR test. Pnoc-CreeNpHR3.0 mice show significantly increased number of rewards received following nosepoke-paired photoinhibition, compared to Controls. (n=6: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepoke-paired inhibition Rewards for Control versus Pnoc-CreeNpHR3.0, ***p < 0.001).
(N) Average number of licks per reward performed during PR test. There is no significant difference in lick rate between Control and Pnoc-CreeNpHR3.0 mice following all stimulation parameters. (n = 6: two-way repeated-measures ANOVA, ns).
Figure S6. Related to Figure 6: Optogenetic and Chemogenetic Stimulation of pnVTAPnoc Neurons Decreases Effort to Receive a Natural Reward
(A) Coronal brain images depicting representative ChR2-eYFP expression following viral injection into the pnVTA of Pnoc-Cre+ mice (AP:−3.3) Image shows 10x (left panel) and 63X (right panel)). Nissl (Blue), Tyrosine hydroxylase (red) and ChR2-eYFP (green).
(B) Sagittal brain image depicting representative ChR2-eYFP expression following viral injection into the pnVTA of Pnoc-Cre+ mice. Nissl (Blue), Tyrosine hydroxylase (red) and ChR2-eYFP (green).
(C) Number of nosepokes performed during PR test during photostimulation time-locked to either nosepokes or licks. Pnoc-CreChR2/eYFP mice show significantly decreased active nosepokes compared to Controls following 60-minute stimulation, but are not different following nosepoke or lick time-locked stimulation. (n = 8: two-way repeated-measures ANOVA, Bonferroni post hoc; *p < 0.05)
(D) Performance-based separation of Pnoc-CreChR2/eYFP mice showing average number of nosepokes performed during PR test and during Baseline (BL), Nosepoke-paired (NP) or Lick-paired (Lick) photostimulation. “Diminished” responding Pnoc-CreChR2/eYFP mice (n=8) show significantly decreased nosepokes compared “Enhanced” responding Pnoc-CreChR2/eYFP (n=4) during nosepoke-paired stimulation. (n = 4 - 8: two-way repeated-measures ANOVA, Diminished NP vs. Lick Stimulation **p < 0.01; Enhanced NP vs. Lick Stimulation *p < 0.05)
(E) Average cumulative nosepokes during PR test for sucrose in Pnoc-CreChR2/eYFP mice (n = 12: two-way repeated-measures ANOVA, Nosepokes for Pnoc-CreChR2/eYFP mice, No Stimulation versus Stimulation, *p < 0.05, ***p < 0.001).
(F) Number of nosepokes (left panel) and rewards (right panel) during FR3 operant conditioning. Pnoc-CreChR2/eYFP mice show reduction in nosepokes and number of rewards received during 10 Hz stimulation compared to baseline and controls (n = 8 to 12, two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Pnoc-CreChR2/eYFP mice, baseline versus stimulation, **p < 0.01; Rewards for Pnoc-CreChR2/eYFP mice, baseline versus stimulation ***p < 0.001
(G) Average number of licks per reward performed during (PR) test. There is no significant difference in lick rate in Pnoc-CreChR2/eYFP mice between no stimulation and stimulation. (n = 12: Student’s t-test, ns).
(H) Percentage of time spent in the stimulation side for different frequencies (5, 10, 20, 60Hz) in control and Pnoc-CreChR2/eYFP mice (n = 9 to 12: two way repeated-measures ANOVA, Bonferroni post hoc; percentage of time spent in stimulation side for control versus ChR2 at (5Hz) *p < 0.05, percentage of time spent in stimulation side for control versus ChR2 at (10Hz) *p < 0.05).
(I) Representative heatmap of relative time spent during real-time place testing for a Pnoc-CreChR2/eYFP mouse that received 5 Hz photostimulation in the stimulation chamber (top) and the same mouse that received no photostimulation in the stimulation chamber (bottom).
(J) Coronal image depicting representative Gq-mCherry expression following viral injection in Pnoc-Cre+ mice. Image shows Tyrosine hydroxylase (green) and Gq-mCherry (red).
(K) Number of rewards for baseline versus CNO (5mg/kg) during PR test **p < 0.01). Control mice show no effect in number of rewards received following CNO (5mg/kg) administration.
(L) Number of rewards during PR test. Pnoc-CrehM3D(Gq) mice show a significant decrease in the number of rewards received following CNO (5mg/kg) administration compared to controls (data presented as mean ± SEM, n = 8 to 11: two way repeated-measures ANOVA, Bonferroni post hoc; Rewards for baseline versus CNO (5mg/kg) during PR test **p < 0.01). Control mice show no effect in number of rewards received following CNO (5mg/kg) administration
(M) Number of rewards (as % of vehicle treatment) during PR test in Pnoc-CrehM3D(Gq) mice that received CNO (1 mg/kg or 5 mg/kg) and the NOPR antagonist (J-113397, 10 mg/kg). CNO treated mice show a significant decrease in rewards compared to controls that was partially rescued by treatment with J-113397. (data presented as mean ± SEM, n = 8 to 11: two way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for baseline versus CNO (5mg/kg) during PR test **p < 0.01, Rewards for baseline versus CNO (5mg/kg) during PR test **p < 0.01).
(N) Distance traveled during Pre-test and Post-test following conditioning paradigm. There is no difference in the distance traveled between control and Pnoc-CrehM3D(Gq) mice.
Figure S7. Related to Figure 7: VTA NOPR Expression is Necessary and Sufficient for Regulating Motivation for Sucrose
(A) Schematic of YFP-tagged NOPR conditional knock-out targeting vector
(B) Representative southern blots for Oprl1 conditional KO (KpnI digest)
(C) Representative traces for J-113397 (top), Vehicle (middle) and SCH-221510 (bottom)
(D) Average lick rate for DAT-Cre mice during Pavlovian conditioning over rewards following vehicle, SCH-221510, or J-113397 administration.
(E) Representative heat map displaying average neural activity for each mouse during Pavlovian conditioning
(F)Average lick rate for control, NOPR KO and NOPR cKO mice during PR test (Two-way repeated measures ANOVA, ns).
(G) Representative lick rate rasters for control, NOPR KO and NOPR cKO mice (H) Pie chart graph depicting %Co-expression Cre and TH (top panel) in the VTA of NOPRlox/lox+ mice that received injections of AAV9-rTH-PI-Cre-SV40 (n=4, 4 slices per n).
(I) Number of nosepokes during PR test session following vehicle and SCH221510 (10mg/kg) administration. TH-Cre x NOPR−/− “rescue” mice show a significant decrease in the number of rewards received following SCH221510 (10mg/kg) administration compared to NOPR−/− mice (n = 8 to 10: two way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for NOPR-rescue mice Vehicle versus SCH221510 (10mg/kg) PR test ***p < 0.001.
(J) Representative lick rate raster for NOPR-rescue mice given either vehicle or SCH-221510 prior to PR test.
(K) Total distanced traveled in an open field assay at baseline, vehicle, and SCH-221510 (10mg/kg) treated NOPR KO X TH-Cre mice that were injected with eYFP or NOPR.
Highlights.
pnVTAPnoc neurons project locally onto VTA dopamine neurons
pnVTAPnoc neurons become active when animals are demotivated to seek rewards
Stimulation of pnVTAPnoc neurons reduces breakpoint and promotes avoidance behavior
VTA dopamine neuron NOPR expression is necessary to limit reward-seeking behavior
Acknowledgements
This work was supported by Mallinckrodt Professorship, Hope Center for Neurological Studies (NIH P30 Blueprint Core, for Viruses), and the NIH (R37R21DA034929, 2T32DA007261-26, T32EB014856). Dougherty lab is supported by NARSAD Independent Investigator grant from the Brain and Behavior Research Foundation, and NIH (5U01MH109133, T32GM008151). Stuber lab is supported by NIH (R37DA032750, R01DA038168 (GDS) and F32DA041184. Support also provided by the Hope Center Viral Vectors Core, and the Genome Technology Resource Center at Washington University in St. Louis (NIH P30CA91842 and UL1TR000448). We also thank Dr. Ilya Monosov (Washington University) and Dr. Paul Philips (U. Washington) for helpful insights and discussion.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL,, Rogers JA, Bruchas MR (2015). Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron, 87, 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ (1996). Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol. 368(2), 229–251. [DOI] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL (2015). Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol, 172(2), 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L (2015). Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input–Output Mapping. Cell, 162(3), 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB (2001). Neurotensin and dopamine interactions. Pharmacol Rev, 53, 453–486. [PubMed] [Google Scholar]
- Castro DC, & Bruchas MR (2019). A Motivational and Neuropeptidergic Hub: Anatomical and Functional Diversity within the Nucleus Accumbens Shell. Neuron, 102(3), 529–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Polidori C, Antonelli L, Salvadori S, Guerrini R, Massi M (2002). Pharmacological characterization of the nociceptin receptor which mediates reduction of alcohol drinking in rats. Peptides, 23(1), 117–25. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N, Phillips PE (2010). Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nature Methods, 7, 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S (2014). Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc. Natl. Acad. Sci. USA, 111, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK (1998). Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends in Neurosciences, 21(5), 215–221. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, D’Souza MS, Potter DN, Chartoff EH, Carlezon WA Jr., Pizzagalli DA, Markou A (2017). Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology, 234, 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing AG and Wightman RM (1984). Monitoring the stimulated release of dopamine with in vivo voltammetry. II: clearance of released dopamine from extracellular fluid. Journal of Neurochemistry, 43, 570–577. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, & Phillips PE (2010). Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nature neuroscience, 13(1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW (2011). Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc Natl Acad Sci USA, 108(3), 15647–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, et al. (2014). Natural neural projection dynamics underlying social behavior. Cell, 157(7), 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AS, Rutledge RB, Glimcher PW & Phillips PE Phasic dopamine release in the rat nucleus accumbens symmetrically encodes a reward prediction error term. J. Neurosci 34, 698–704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W (1961). Progressive ratio as a measure of reward strength. Science, 134(3483), 943–944. [DOI] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, & Hikosaka O (2011). Negative reward signals from lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. The Journal of Neuroscience, 31(32), 11457–11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, & Holland PC (2009). The rostromedial tegmental nucleus (RMTg), a major GABAergic afferent to midbrain dopamine neurons, selectively encodes aversive stimuli and promotes behavioral inhibition. Neuron, 61(5), 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W (2008). Influence of reward delays on responses of dopamine neurons. J Neurosci 28:7837–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT (2001). Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl), 154(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ (2003). Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. Journal of Neuroscience, 23(32), 10331–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience, 13(1), 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment NT, Chen Y, Tan AM, Murphy NP, Leslie FM (2002). Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport, 13(9), 1137–40. [DOI] [PubMed] [Google Scholar]
- Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, Wildes CP, Ungless MA, Tye KM (2016). Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell, 164(4), 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR (2015). CRH engagement of the Locus Coeruleus Noradrenergic System mediates Stress-Induced anxiety, Neuron, 87, 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC (1994). ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett, 341, 33–38. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Mouledous L (2000). Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides, 21(7), 907–917. [DOI] [PubMed] [Google Scholar]
- Morales M, Margolis EB (2017). Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nature Reviews Neuroscience, 18, 73–85. [DOI] [PubMed] [Google Scholar]
- Mulvey B, Bhatti DL, Gyawali S, Lake AM, Kriaucionis S, Ford CP, Bruchas MR, Heintz N, Dougherty JD (2018). Molecular and Functional Sex Differences of Noradrenergic Neurons in the Mouse Locus Coeruleus. Cell Reports, 23(8), 2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT (1999). Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem 73, 179–186. [DOI] [PubMed] [Google Scholar]
- Namburi P, Al-Hasani R, Calhoon GG, Bruchas MR, & Tye KM (2016). Architectural representation of valence in the limbic system. Neuropsychopharmacology, 41(7), 1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, & Roeper J (2002). Ih channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. Journal of Neuroscience, 22(4), 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, … & Yamashita T (1997). Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. The EMBO Journal, 16(8), 1858–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton CS, Neal CR, Kumar S, Akil H, Watson SJ (2002). Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neuro, 444, 358–368. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Billington CJ, Levine AS (2000). Fos expression in feeding-related brain areas following intracerebroventricular administration of orphanin FQ in rats. Brain Research, 855, 171–175. [DOI] [PubMed] [Google Scholar]
- Ozawa A, Brunori G, Mercatelli D, Wu J, Cippitelli A, Zou B, Xie XS, Williams M, Zaveri NT, Low S, Scherrer G, Kieffer BL, Toll L (2015). Knock-In Mice with NOP-eGFP Receptors Identify Receptor Cellular and Regional Localization. J Neurosci, 35(33), 11682–11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva I & Wanat MJ (2019) Operant Costs Modulate Dopamine Release to Self-Administered Cocaine. The Journal of Neuroscience 39, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker NF, Cameron CM, Taliaferro JP, Lee J, Choi JY, Davidson TJ, Daw ND, Witten I (2016). Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nature Neuroscience, 19(6), 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, Folk RW, Broussard GJ, Liang R, Jang MJ, Tian L (2018). Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensor. Science, 360 eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologruto TA, Yasuda R, Svoboda K (2004). Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J Neurosci. 24(43), 9572–9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, O'Brien D, Pisat N, Weichselbaum CT, Sakers K, Lisci M, Dalal JS, Dougherty JD (2017). A Comprehensive Analysis of Cell Type-Specific Nuclear RNA From Neurons and Glia of the Brain. Biol Psychiatry, 81(3), 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods, 66(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Rodi D, Polidori C, Bregola G, Zucchini S, Simonato M, Massi M (2002). Pronociceptin/orphanin FQ and NOP receptor mRNA levels in the forebrain of food deprived rats. Brain Research, 957(2), 354–361. [DOI] [PubMed] [Google Scholar]
- Russo SJ, & Nestler EJ (2013). The Brain Reward Circuitry in Mood Disorders. Nature Reviews. Neuroscience, 14(9), 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakers K, Lake AM, Khazanchi R, Ouwenga R, Vasek MJ, Dani A, Dougherty JD (2017). Astrocytes locally translate transcripts in their peripheral processes. Proc Natl Acad Sci USA, 114, E3830–E3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ & Pardo M Dopamine, behavioral economics, and effort. Front. Behav. Neurosci 3, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W Dayan P Montague PR (1997) A Neural Substrate of Prediction and Reward. Science, 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Schultz W (2007). Multiple dopamine functions at different time courses. Annual Reviews in Neuroscience, 30, 259–288. [DOI] [PubMed] [Google Scholar]
- Seo D, Funderburk SC, Bhatti DL, Motard LE, Newbold D, Girven KS, McCall JG, Krashes M, Sparta DR, Bruchas MR (2016). A GABAergic Projection from the Centromedial Nuclei of the Amygdala to Ventromedial Prefrontal Cortex Modulates Reward Behavior. The Journal of Neuroscience, 36(42), 10831–10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks, Genome Research, 13(11), 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, Sousa N, Rodrigues AJ (2016). Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun, 7, 11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ, Funderburk SC, McCall JG, Gereau RW, Bruchas MR (2015). Spatiotemporal control of opioid signaling and behavior. Neuron, 86, 923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD (2012). Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nature Neuroscience, 15, 1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer W,R,, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, Schultz W (2016). Dopamine neuron-specific optogenetic stimulation in Rhesus macaques. Cell, 166, 1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Holahan MR, Kelley AE (1997). Injections of nociceptin into nucleus accumbens shell or ventromedial hypothalamic nucleus increase food intake. Neuroreport, 8, 423–426. [DOI] [PubMed] [Google Scholar]
- Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, … Li Y (2018). A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell, 174(2), 481–496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C (2012). GABA neurons of the VTA drive conditioned place aversion. Neuron, 73, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, et al. (2011). A Resource of Cre Driver Lines for Genetic Targeting of GABAergic Neurons in Cerebral Cortex. Neuron, 71(6), 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DG et al. (2016). A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron, 92(2), 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Huang R, Cohen JY, Osakada F, Kobak D, Machens CK, Callaway EM, Uchida N, Watabe-Uchida M (2016). Distributed and mixed information in monosynaptic inputs to dopamine neurons. Neuron, 91(6), 1374–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo’ G, Cox BM, & Zaveri NT (2016). Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacological Reviews, 68(2), 419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA & Stuber GD (2012). Activation of VTA GABA neurons disrupts reward consumption. Neuron, 73, 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, & Baler RD (2011). Reward, dopamine and the control of food intake: implications for obesity. Trends in Cognitive Sciences, 15(1), 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A & Uchida N (2012). Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron, 74, 858–873. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R (2014). The biology of nociceptin/orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther 141, 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT (2011). The Nociceptin/Orphanin FQ Receptor (NOP) as a Target for Drug Abuse Medications. Current Topics in Medicinal Chemistry, 11(9), 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Grandy DK, Johnson SW (2002). Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharmacol. 136(7), 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW (2003). Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport, 14(18), 2383–2385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Related to STAR Methods Section: Stereotaxic viral / optical fiber implant surgeries. List of viruses used in stereotaxic surgery
Table S2. Related to Figure 4: pnVTAPnoc TRAP: Genes of Interest
Movie S1. Related to Figure 2: pnVTAPnoc Neural Activity During Breakpoint
Figure S1. Related to Figure 1: Anatomical Identification of Endogenous Pnoc-expressing VTA Inputs
(A) Schematic of targeting vector for generation of Pnoc-Cre mouse line.
(B) Representative southern blot of Pnoc-Cre knock-in
(C) Quantification of Cre expression within Pnoc-expressing cells in the BNST (1988/2122 cells), CeA (786/805 cells), and pnVTA (435/450 cells). (n=4, 4 mice, 3 slices each)
(D) Confirmation and validation of Pnoc-Cre mouse line depicting coronal images for Pnoc in Pnoc-Cre- mice for in situ hybridization colocalization of Pnoc (green) and Cre (red) for coronal images of BNST, CeA, and IPN/VTA regions.
(E) Confirmation of Pnoc-labeling within coronal images of the dentate gyrus (DG) of the hippocampus from left to right of: Pnoc mRNA labeling from mouse Allen Brain Atlas, RNAscope in situ hybridization of DAPI (blue) and Pnoc mRNA (green), Pnoc (red) labeling in Pnoc-Cre x Ai9-tdTomato against Nissl (blue) immunostaining, and Pnoc (green) labeling in Pnoc-Cre × Ai32-ChR2/eYFP against Nissl (blue).
(F) Confirmation of Pnoc-labeling within coronal images of the septum from left to right of: Pnoc mRNA labeling from mouse Allen Brain Atlas, RNAscope in situ hybridization of DAPI (blue) and Pnoc mRNA (green), Pnoc (red) labeling in Pnoc-Cre × Ai9-tdTomato against Nissl (blue) immunostaining, and Pnoc (green) labeling in Pnoc-Cre × Ai32-ChR2/eYFP against Nissl (blue).
(G) Coronal images depicting cre-dependent helper (red channel, left panel) and monosynaptic rabies (green channel, right panel) viral expression in the VTA / IPN regions of DAT-Cre mice corresponding to Figure 1F. PBP- parabrachial pigmented nucleus. Representative image from n=3.
(H) Coronal images that correspond to Figure 1H showing GFP (red, right panel) and Pnoc (green, left panel) mRNA following recombinant AAV helper viral and rabies viral injections into the VTA of DAT-Cre mice PN-paranigral, PBP- parabrachial pigmented nucleus. Representative images from n=3.
(I) Cartoon depicting recombinant retrograde AAV2 virus injection into the VTA of Pnoc-Cre mice. Coronal image depicts GFP (green) Pnoc+ cells in the paranigral (PN) region of the VTA against DAPI (blue) and tyrosine hydroxylase (red) immunostaining. PBP- parabrachial pigmented nucleus. Representative images taken from n=3.
(J) In situ hybridization colocalization of Pnoc (green) and DAT (red) for coronal images of zoomed VTA regions. Closed arrows represent colocalization of Pnoc and DAT, and empty arrows represent Pnoc labeled cells that are absent of DAT.
(K) Pie chart depicting quantification of Pnoc and DAT colocalization within the paranigral VTA (pnVTA) (n=3).
Figure S2. Related to Figure 2: Pnoc+ VTA Inputs are Engaged During Low-Yield Reward-Seeking
(A) Raw transduced photon emissions from excitation with 405 nm light and 470 nm light.
(B) Double exponential curve (red line) fit to signal after subtraction of 405 nm channel from 470 nm channel.
(C) Resulting baseline-corrected signal after subtraction of double exponential curve.
(D) Maximum activity (maximum 0.5 seconds of activity prior to reward epoch) for each FR3 level (n = 18 mice, 33 sessions: Pearson’s correlation, *p = 0.033, r = −0.073).
(E) Maximum activity (maximum 0.5 seconds of activity prior to reward epoch) relative to nosepoke criterion for each PR level (n = 18 mice, 32 sessions: Pearson’s correlation, ***p<0.001, r = 0.320).
(F) Mean neural activity during active nosepoking (see Figure 2H) relative to proportion of nosepokes until breakpoint for each PR session. Same data as Figure 2G, but split based on anatomical location of fiber implant within VTA (see Figure 3). (n = 18 mice, 32 sessions; Two-way repeated measures ANOVA: interaction, F = 0.49, p = 0.883).
Figure S3. Related to Figure 3: Anatomically Distinct Subdivisions of VTA Pnoc+ Neurons are Engaged Differentially During Reward Anticipation and Consumption
(A) Immunohistochemistry for GCaMP6s and tyrosine hydroxylase (TH) staining in Pnoc-Cre mice. Coronal sections show GCaMP6s (green), TH (red) and fiber implant tract (yellow) at various anterior/posterior coordinates throughout VTA.
(B) Time intervals relative to reward period that were used to calculate mean neural activity in Figure 3B. Trace of mean neural activity (red) taken from Figure 3K (middle panel) for reference.
(C) Immunohistochemistry for GCaMP6s and tyrosine hydroxylase (TH) staining in DAT-Cre mice. Coronal sections show GCaMP6s (green), TH (red) and fiber implant tract (yellow) at representative implant site.
(D) Coronal section cartoon showing anatomical location of 400μm optic fiber implant placement for DAT-Cre mice.
(E) Raster plot depicting reward consumption (lick rate) during a representative FR3 task session with simultaneous fiber photometry recording.
(F) Raster plot depicting reward consumption (lick rate) during a representative PR task session with simultaneous fiber photometry recording.
Figure S4. Related to Figure 4: pnVTAPnoc Neurons are molecularly heterogeneous
(A) Representative coronal images of DAPI (blue), Pnoc (green) and VGAT (red) mRNA expression within the pnVTA as a progression from anterior to posterior (AP). Open arrows represent no colabeling and filled-in arrows represent colabeling.
(B) Representative coronal images of DAPI (blue), Pnoc (green), VGlut2 (purple) mRNA expression within the pnVTA as a progression from anterior to posterior (AP). Open arrows represent no colabeling, and filled-in arrows represent colabeling.
(C) Scatterplot representing enriched mRNA (10 Fold) in pnVTAPnoc neurons. Pnoc presented in blue.
(D) Representative coronal image showing immunohistochemistry for AAV8-bacTRAP-eYFP and tyrosine hydroxylase (TH) staining. Images show bacTRAP (green), and TH (red).
(E) Representative VTA ChR2 expression patterns in vGlut2-Cre and vGAT-Cre mice.
Figure S5. Related to Figure 5; Selective Ablation and Inhibition of pnVTAPnoc Neurons Enhances Operant Responding for Natural Rewards
(A) Representative coronal images of the pnVTA and IPN showing in situ hybridization for nociceptin (N/OFQ) mRNA following viral injection in Pnoc-Cre− (left panel) and Pnoc-Cre+ (right panel) mice. Images show N/OFQ (green).
(B) Number of nosepokes (left panel) and rewards (right panel) across 3 days of FR1 and 3 days of FR3 sucrose self-administration. Pnoc-CretaCasp3 mice show significantly increased nosepokes and rewards received, compared to controls (n = 11 to 13: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Control versus Casp3 on day 2 and 3 of FR3 training *p < 0.05, Rewards for control versus Casp3 on day 2 and 3 of FR3 training *p < 0.05).
(C) Cumulative nosepokes across 60 min session Pnoc-Cre+ and Pnoc-Cre− mice that received injections of AAV2-FLEX-taCasp3-TEVp. (n = 11 to 13: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Control versus Casp3 **p < 0.05, **p < 0.01).
(D) Total nosepokes for each 10 min epoch across 60 min session in Pnoc-Cre+ and Pnoc-Cre− mice that received injections of AAV2-FLEX-taCasp3-TEVp. (n = 11 to 13: two-way repeated-measures ANOVA, ns).
(E) Average number of rewards received during PR test. Pnoc-CretaCasp3 mice show significantly increased number of rewards received, compared to controls (n = 11 to 13: unpaired t-test, Rewards for Control versus Casp3 during PR test *p < 0.05).
(F) Average number of licks per reward performed during PR test. Pnoc-CretaCasp3 mice mice show increased number of licks per reward received compared to Controls (n = 11 to 13: unpaired t-test, licks per reward for Control versus Casp3 during PR test, p = 0.08).
(G) Cumulative nosepokes across 60 min session of Pnoc-CrehM4D(Gi) given vehicle or CNO (5mg/kg). Pnoc-CrehM4D(Gi) mice show significantly increased number of cumulative nosepokes following CNO administration, compared to Vehicle. (n = 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Vehicle versus CNO, * p < 0.05, *** p < 0.001).
(H) Total nosepokes for each 10 min epoch across 60 min session of Pnoc-CrehM4D(Gi) mice given vehicle or CNO (5mg/kg). (n = 8: two-way repeated-measures ANOVA, ns).
(I) Average number of rewards received during PR test. Pnoc-CrehM4D(Gi) mice show significantly increased number of rewards received following CNO (5mg/kg administration), compared to vehicle treatment. (n = 8: two-way repeated-measures ANOVA, Bonferroni post hoc; Rewards for Vehicle versus 5mg/kg, ***p < 0.001, 1mg/kg versus 5mg/kg **p < 0.01).
(J) Average number of licks per reward performed during PR test. There is no significant difference in lick rate between Control and Pnoc-CrehM4D(Gi) mice following administration of all doses of CNO (n=8: two-way repeated-measures ANOVA, ns).
(K) Cumulative nosepokes across 60 min session of Pnoc-CreeNpHR3.0 mice given either no inhibition or nosepoke-paired photoinhibition. Pnoc-CreeNplHR3.0 mice show significantly increased number of cumulative nosepokes following nosepoke-paired photoinhibition, compared to Baseline. (n=6: two-way repeated-measures ANOVA, Bonferroni post hoc; Cumulative nosepokes for Baseline versus Nosepoke, *p < 0.05, **p < 0.01).
(L) Total nosepokes for each 10 min epoch across 60 min session for Pnoc-CreeNpHR3.0 mice given either no inhibition or nosepoke-paired photoinhibition. (n = 6: two-way repeated-measures ANOVA, ns).
(M) Average number of rewards received during PR test. Pnoc-CreeNpHR3.0 mice show significantly increased number of rewards received following nosepoke-paired photoinhibition, compared to Controls. (n=6: two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepoke-paired inhibition Rewards for Control versus Pnoc-CreeNpHR3.0, ***p < 0.001).
(N) Average number of licks per reward performed during PR test. There is no significant difference in lick rate between Control and Pnoc-CreeNpHR3.0 mice following all stimulation parameters. (n = 6: two-way repeated-measures ANOVA, ns).
Figure S6. Related to Figure 6: Optogenetic and Chemogenetic Stimulation of pnVTAPnoc Neurons Decreases Effort to Receive a Natural Reward
(A) Coronal brain images depicting representative ChR2-eYFP expression following viral injection into the pnVTA of Pnoc-Cre+ mice (AP:−3.3) Image shows 10x (left panel) and 63X (right panel)). Nissl (Blue), Tyrosine hydroxylase (red) and ChR2-eYFP (green).
(B) Sagittal brain image depicting representative ChR2-eYFP expression following viral injection into the pnVTA of Pnoc-Cre+ mice. Nissl (Blue), Tyrosine hydroxylase (red) and ChR2-eYFP (green).
(C) Number of nosepokes performed during PR test during photostimulation time-locked to either nosepokes or licks. Pnoc-CreChR2/eYFP mice show significantly decreased active nosepokes compared to Controls following 60-minute stimulation, but are not different following nosepoke or lick time-locked stimulation. (n = 8: two-way repeated-measures ANOVA, Bonferroni post hoc; *p < 0.05)
(D) Performance-based separation of Pnoc-CreChR2/eYFP mice showing average number of nosepokes performed during PR test and during Baseline (BL), Nosepoke-paired (NP) or Lick-paired (Lick) photostimulation. “Diminished” responding Pnoc-CreChR2/eYFP mice (n=8) show significantly decreased nosepokes compared “Enhanced” responding Pnoc-CreChR2/eYFP (n=4) during nosepoke-paired stimulation. (n = 4 - 8: two-way repeated-measures ANOVA, Diminished NP vs. Lick Stimulation **p < 0.01; Enhanced NP vs. Lick Stimulation *p < 0.05)
(E) Average cumulative nosepokes during PR test for sucrose in Pnoc-CreChR2/eYFP mice (n = 12: two-way repeated-measures ANOVA, Nosepokes for Pnoc-CreChR2/eYFP mice, No Stimulation versus Stimulation, *p < 0.05, ***p < 0.001).
(F) Number of nosepokes (left panel) and rewards (right panel) during FR3 operant conditioning. Pnoc-CreChR2/eYFP mice show reduction in nosepokes and number of rewards received during 10 Hz stimulation compared to baseline and controls (n = 8 to 12, two-way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for Pnoc-CreChR2/eYFP mice, baseline versus stimulation, **p < 0.01; Rewards for Pnoc-CreChR2/eYFP mice, baseline versus stimulation ***p < 0.001
(G) Average number of licks per reward performed during (PR) test. There is no significant difference in lick rate in Pnoc-CreChR2/eYFP mice between no stimulation and stimulation. (n = 12: Student’s t-test, ns).
(H) Percentage of time spent in the stimulation side for different frequencies (5, 10, 20, 60Hz) in control and Pnoc-CreChR2/eYFP mice (n = 9 to 12: two way repeated-measures ANOVA, Bonferroni post hoc; percentage of time spent in stimulation side for control versus ChR2 at (5Hz) *p < 0.05, percentage of time spent in stimulation side for control versus ChR2 at (10Hz) *p < 0.05).
(I) Representative heatmap of relative time spent during real-time place testing for a Pnoc-CreChR2/eYFP mouse that received 5 Hz photostimulation in the stimulation chamber (top) and the same mouse that received no photostimulation in the stimulation chamber (bottom).
(J) Coronal image depicting representative Gq-mCherry expression following viral injection in Pnoc-Cre+ mice. Image shows Tyrosine hydroxylase (green) and Gq-mCherry (red).
(K) Number of rewards for baseline versus CNO (5mg/kg) during PR test **p < 0.01). Control mice show no effect in number of rewards received following CNO (5mg/kg) administration.
(L) Number of rewards during PR test. Pnoc-CrehM3D(Gq) mice show a significant decrease in the number of rewards received following CNO (5mg/kg) administration compared to controls (data presented as mean ± SEM, n = 8 to 11: two way repeated-measures ANOVA, Bonferroni post hoc; Rewards for baseline versus CNO (5mg/kg) during PR test **p < 0.01). Control mice show no effect in number of rewards received following CNO (5mg/kg) administration
(M) Number of rewards (as % of vehicle treatment) during PR test in Pnoc-CrehM3D(Gq) mice that received CNO (1 mg/kg or 5 mg/kg) and the NOPR antagonist (J-113397, 10 mg/kg). CNO treated mice show a significant decrease in rewards compared to controls that was partially rescued by treatment with J-113397. (data presented as mean ± SEM, n = 8 to 11: two way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for baseline versus CNO (5mg/kg) during PR test **p < 0.01, Rewards for baseline versus CNO (5mg/kg) during PR test **p < 0.01).
(N) Distance traveled during Pre-test and Post-test following conditioning paradigm. There is no difference in the distance traveled between control and Pnoc-CrehM3D(Gq) mice.
Figure S7. Related to Figure 7: VTA NOPR Expression is Necessary and Sufficient for Regulating Motivation for Sucrose
(A) Schematic of YFP-tagged NOPR conditional knock-out targeting vector
(B) Representative southern blots for Oprl1 conditional KO (KpnI digest)
(C) Representative traces for J-113397 (top), Vehicle (middle) and SCH-221510 (bottom)
(D) Average lick rate for DAT-Cre mice during Pavlovian conditioning over rewards following vehicle, SCH-221510, or J-113397 administration.
(E) Representative heat map displaying average neural activity for each mouse during Pavlovian conditioning
(F)Average lick rate for control, NOPR KO and NOPR cKO mice during PR test (Two-way repeated measures ANOVA, ns).
(G) Representative lick rate rasters for control, NOPR KO and NOPR cKO mice (H) Pie chart graph depicting %Co-expression Cre and TH (top panel) in the VTA of NOPRlox/lox+ mice that received injections of AAV9-rTH-PI-Cre-SV40 (n=4, 4 slices per n).
(I) Number of nosepokes during PR test session following vehicle and SCH221510 (10mg/kg) administration. TH-Cre x NOPR−/− “rescue” mice show a significant decrease in the number of rewards received following SCH221510 (10mg/kg) administration compared to NOPR−/− mice (n = 8 to 10: two way repeated-measures ANOVA, Bonferroni post hoc; Nosepokes for NOPR-rescue mice Vehicle versus SCH221510 (10mg/kg) PR test ***p < 0.001.
(J) Representative lick rate raster for NOPR-rescue mice given either vehicle or SCH-221510 prior to PR test.
(K) Total distanced traveled in an open field assay at baseline, vehicle, and SCH-221510 (10mg/kg) treated NOPR KO X TH-Cre mice that were injected with eYFP or NOPR.
Data Availability Statement
RNA sequencing data for pnVTAPnoc neurons from Figure 4 have been deposited and are available from GEO (Accession: GSE108813). Custom MATLAB analysis code was created to appropriately organize, process, and combine photometry recording data with associated behavioral data. Analysis code for photometry from Figure 2 and 3 is available online at: www.github.com/BruchasLab. The full behavioral data set supporting the current study are available from the corresponding author upon request.