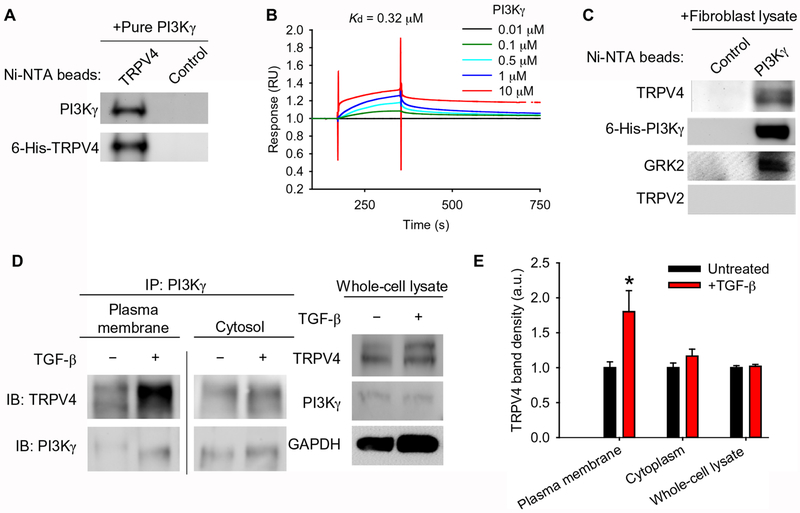
Fig. 2. TRPV4 and PI3Kγ interact directly and translocate to the plasma membrane in response to TGF-β.
(A) The binding of purified PI3Kγ to purified 6-His-TRPV4 coupled to Ni-NTA beads was assessed by immunoblotting with an antibody specific for PI3Kγ. The blots were stripped and reprobed with an antibody for TRPV4. Control indicates beads alone (no protein bound to beads), N=3 independent experiments. (B) The direct interaction between immobilized purified 6-His-TRPV4 and purified 6-His-PI3Kγ was measured by surface plasmon resonance. N=3 independent experiments. (C) The binding of purified 6-His-PI3Kγ coupled to Ni-NTA beads to endogenous TRPV4 from human lung fibroblast (HLF) lysates was assessed by Western blotting. Blots were stripped and reprobed with antibodies specific for PI3Kγ, GRK2 (positive control), and TRPV2 (negative control). Control indicates beads alone (no protein bound to beads), N=3 independent experiments. (D) PI3Kγ immunoprecipitates (IP) from plasma membrane and cytosolic fractions of HLFs treated with TGF-β as indicated were immunoblotted for PI3Kγ and TRPV4. The line between the plasma membrane and cytosolic fractions indicates non-contiguous lanes from the same blot. Total whole cell lysate of cells treated ±TGF-β (input) was immunoblotted for TRPV4 and PI3Kγ. GAPDH is a loading control. (E) Quantification of TRPV4 in plasma membrane and cytosolic fractions normalized to TRPV4 in whole cell lysate as in (D). Data represent means ± SEM. N=3 independent experiments. *P≤0.05 between untreated and TGF-β conditions, using ANOVA followed by Student-Newman-Keuls multiple comparisons test.