Fig. 1. Carbon and nitrogen oxidation states and biology.
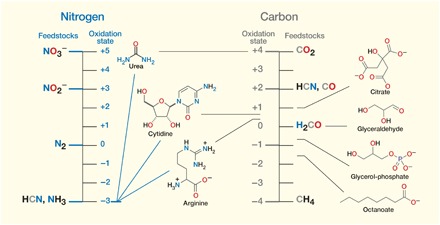
Nitrogen in biological molecules is predominantly in the −3 oxidation state, whereas carbon is in a broader range roughly centering on 0. Thus, if methane (CH4) were to have been used as an initial carbon feedstock, oxidation chemistry would have been required. Similarly, carbon dioxide (CO2) would have required reduction. In light of just the foregoing, hydrogen cyanide (HCN) can be seen as a near-ideal feedstock, as it provides both nitrogen in approximately the right oxidation state and carbon only in need of some reduction. This partial reduction actually turns out to be necessary for the C─C bond construction chemistry required to link carbon atoms into the linear and branched chains so often found in biological molecules. During cyanosulfidic chemistry, these bonds can be made highly efficiently by the addition of HCN to aldehydic reduction products of itself, or related nitriles, to give cyanohydrins [R2C(OH)CN], which can be further reduced and homologated (i.e., add a constant unit, often −CH2−) by the addition of more HCN. Hydrogen cyanide, along with derivatives produced through environmental processing, can be converted by cyanosulfidic chemistry into all the compounds shown (for those which are chiral, in racemic form).
