Fig. 2. Creating stockpiles of initial compounds for prebiotic chemistry.
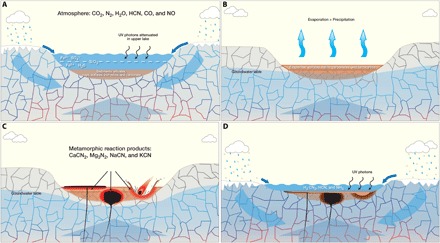
Conceptual model for accumulation of strata in shallow subaqueous basins and their interaction with water and the atmosphere is shown. The model is based on early Mars, but we expect it to be applicable to prebiotic Earth. (A) Lake or shallow sea in contact with bedrock and the atmosphere. Water is supplied by surface runoff from precipitation and/or melting ice, groundwater infiltration from adjacent highlands, and thermally buoyant deep basinal waters warmed by the ambient geothermal gradient and flowing upward through permeable fractures in the crust. HCN in the atmosphere (due to impact delivery and processing) interacts with dissolved iron in the water body and below the depth of UV penetration allows ferrocyanide to form. (B) Variations in climate result in episodic drying of the shallow water body, creating a variety of salts including ferrocyanide. Preservation of ferrocyanide is enhanced by burial beneath the reach of UV energy. (C) Thermal pulses created by igneous intrusions, volcanic activity, or large impacts cause contact metamorphism of cyanide salt deposits to a variety of reaction products including CaCN2, KCN, Mg3N2, and NaCN. (D) Exposure of these metamorphic reaction products to neutral pH water yields H2CN2, HCN, and NH3 in solution. In turn, if these species are exposed to SO2 and surface water is shallow enough to be affected by mid-range UV radiation, cyanosulfidic chemistry can then produce a diverse set of products, which correspond to the nucleotide, amino acid, and lipid precursor molecules of extant biochemistry.
