Fig. 4. Hu1B7 and hu1B7-YTE administration reduces pertussis mortality, leukocytosis, and clinical symptoms in neonatal baboons.
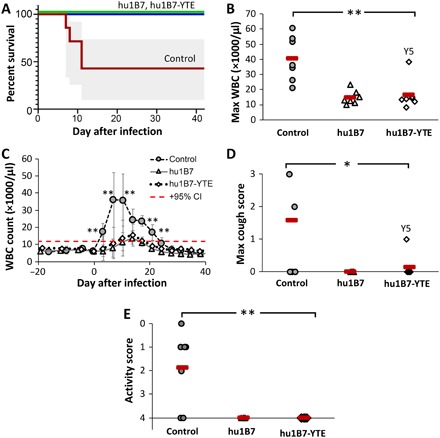
(A) All antibody-treated animals survived infection, while four of seven controls required euthanasia [95% confidence intervals (CI) in gray; P = 0.0023 for each treated group versus controls; log-rank test]. (B) The maximum WBC count was significantly reduced in antibody-treated baboons relative to controls (**P < 0.01; ANOVA, α = 0.05, P = 0.0003; post hoc comparison with Tukey post hoc test). Hu1B7-treated animal T2 was excluded from this analysis on the basis of the Grubb’s test for outliers with α = 0.05. (C) Leukocytosis was delayed and suppressed for antibody-treated animals (group averages ± SD shown; data for individual animals in fig. S2). The control group WBC was significantly higher than treated groups on days indicated (**P < 0.01), and the onset of leukocytosis was delayed from day 3 for control animals to days 10 to 14 for treated animals (P < 0.01). The dashed red line indicates the upper 95% CI for preinfection WBC and defines WBC elevation. (D) Clinical signs showed reduced maximum cough count (0, none; 1, occasional; 2, frequent; and 3, severe and frequent) with antibody treatment (*P < 0.05 control versus either treated group; ANOVA, α = 0.05, P = 0.005; post hoc comparison with Tukey post hoc test) and (E) improved activity scores (0, immobile and requires euthanasia; 1, very little activity; 2, reduced movement and no jumping/climbing; 3, movement but reduced jumping/climbing; and 4, normal activity) for treated versus control animals (**P < 0.01; ANOVA, α = 0.05, P = 0.005; post hoc comparison with Tukey post hoc test).
