Abstract
Background
Tumor infiltrating lymphocytes (TILs) are associated with benefit to trastuzumab and chemotherapy in early-stage HER2+ breast cancer (BC) patients. The predictive value of TILs, TIL subsets, and other immune cells in patients receiving chemotherapy-sparing lapatinib plus trastuzumab (LT) treatment is unclear.
Experimental Design
H&E-stained slides (n=59) were used to score stromal (s-)TILs from pre-treatment biopsies of patients enrolled in the neoadjuvant TBCRC006 trial of 12-week LT therapy (plus endocrine therapy for ER+ tumors). A 60% threshold was used to define lymphocyte-predominant BC (LPBC). Multiplexed immunofluorescence (m-IF) staining (CD4, CD8, CD20, CD68, and FoxP3) and multispectral imaging were performed to characterize immune infiltrates in single FFPE slides (n=33).
Results
The pathologic complete response (pCR) rate was numerically higher in LPBC patients compared to non-LPBC patients (50% vs. 19%, P=0.057). Unsupervised hierarchical clustering of the five immune markers identified two patient clusters with different responses to LT treatment (pCR = 7% vs. 50%, for Cluster 1 vs. 2 respectively, P=0.01). In multivariable analysis, Cluster 2, characterized by high CD4+, CD8+, CD20+ s-TILs, and high CD20+ intratumoral TILs, was independently associated with a higher pCR rate (P=0.03). Analysis of single immune subpopulations revealed a significant association of pCR with higher baseline infiltration by s-CD4, intratumoral (i-) CD4, and i-CD20+ TILs.
Conclusions
LPBC was marginally associated with higher pCR rate than non-LPBC in LT treated HER2+ BC patients. Quantitative assessment of the immune infiltrate by m-IF is feasible and may help correlate individual immune cell subpopulations and immune cell profiles with treatment response.
Keywords: trastuzumab, lapatinib, HER2, TILs, breast cancer, multiplexed immunofluorescence
Background
We and others have shown that a chemotherapy-sparing neoadjuvant therapy regimen containing dual anti-HER2 therapy with lapatinib plus trastuzumab (LT) can achieve pathological complete response (pCR) in as many as one-third of patients with HER2-positive (HER2+) breast cancer (1–3). De-escalation of treatment may decrease toxicity and cost in a substantial number of patients without compromising the clinical outcomes. Germane to the success of de-escalation approaches is the accurate selection of patients who are likely to benefit from such a strategy. Identifying major determinants of sensitivity and resistance to neoadjuvant LT therapy can aid in distinguishing patients who can be optimally treated with this regimen from those who should also receive chemotherapy in addition to anti-HER2 agents (4).
The immune microenvironment of a tumor has recently emerged as a potential modulator of response to treatment and cancer progression (5, 6). Tumor-infiltrating lymphocytes (TILs) are mononuclear immune cells that infiltrate tumor tissue and have been described in most types of solid tumors, including breast cancer (5, 6). In the neoadjuvant setting, while high levels of TILs at baseline are associated with increased pCR rates and/or better event-free survival in HER2+ breast cancer patients in some trials (7–9), these reports are not consistent in all studies (10–14). In some studies, TILs were significantly associated with pCR in univariate but not multivariate analysis (11, 12). These somewhat conflicting results about the predictive value of TILs in HER2+ breast cancer may be attributed either to the lack of an appropriate quantitative approach and/or a standard cut-off for TILs, or to the differences in the treatment regimens among the studies. Since most of these neoadjuvant clinical trials treated patients with chemotherapy in combination with different anti-HER2 agents, the predictive value of TILs for chemotherapy-sparing anti-HER2 therapy in HER2+ breast cancer patients is still unclear.
TILs are usually detected using standard hematoxylin and eosin (H&E)-stained tissue sections under light microscopy and classified as either stromal-TILs (s-TILs) or intratumoral-TILs (i-TILs) (5, 15), following the International TILs Working Group guidelines (15). S-TILs are the lymphocytes present in tumor stroma, whereas i-TILs are those in direct contact with the tumor epithelial cells (15). In breast cancer, s-TILs have been demonstrated to be the predominant population and are considered a more suitable predictive and prognostic marker compared to i-TILs (15). Although the evaluation of TILs by standard H&E staining is simple, inexpensive, and to some extent reproducible, limitations of this approach include its semi-quantitative nature, and its inability to discriminate immune cell subtypes and their spatial organization within the tumoral architecture. The limitations of manual TILs assessment may be overcome by immunohistochemistry (IHC) /immunofluorescence (IF) staining and digital image analysis, which can provide a more accurate measurement and phenotyping of immune cells in tissue sections.
In this study, we measured s-TILs in baseline tumor samples from patients on the TBCRC 006 trial, treated with neoadjuvant LT with endocrine therapy in ER-positive cases for 12 weeks, by the standard H&E-based method, and investigated their role in predicting response to neoadjuvant anti-HER2 treatment without chemotherapy. We also employed an advanced multispectral imaging-based technology in a subset of tumor samples, to characterize stromal and intra-tumoral immune cell subpopulations in a quantitative manner and to define their association with treatment outcome.
Materials and Methods
Patients and specimens
The phase II neoadjuvant clinical trial TBCRC 006 (1) is a multicenter single-arm study conducted through the Translational Breast Cancer Research Consortium (Figure 1). Institutional review board and scientific committee approval were obtained at the lead site (Baylor College of Medicine) and other participating sites. Written informed consent was obtained from all patients. This study was performed in compliance with the Declaration of Helsinki and all applicable US Federal and international regulations and ethical standards. Sixty-six patients with stage II/III HER2+ invasive breast cancer were enrolled, and 64 were eligible and evaluable for response. HER2 positivity was assessed by IHC or fluorescence in situ hybridization (FISH), according to the 2007 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines (16). Patients were treated for 12 weeks with LT [lapatinib 1000 mg orally every day and trastuzumab 4 mg/kg loading dose followed by 2 mg/kg per week]. Patients also received the aromatase inhibitor letrozole 2.5 mg orally once per day (combined with an LHRH agonist of choice in premenopausal women) if their tumor was estrogen receptor (ER) and/or progesterone receptor (PR) positive by IHC (according to ASCO/CAP 2010 guidelines (17)).
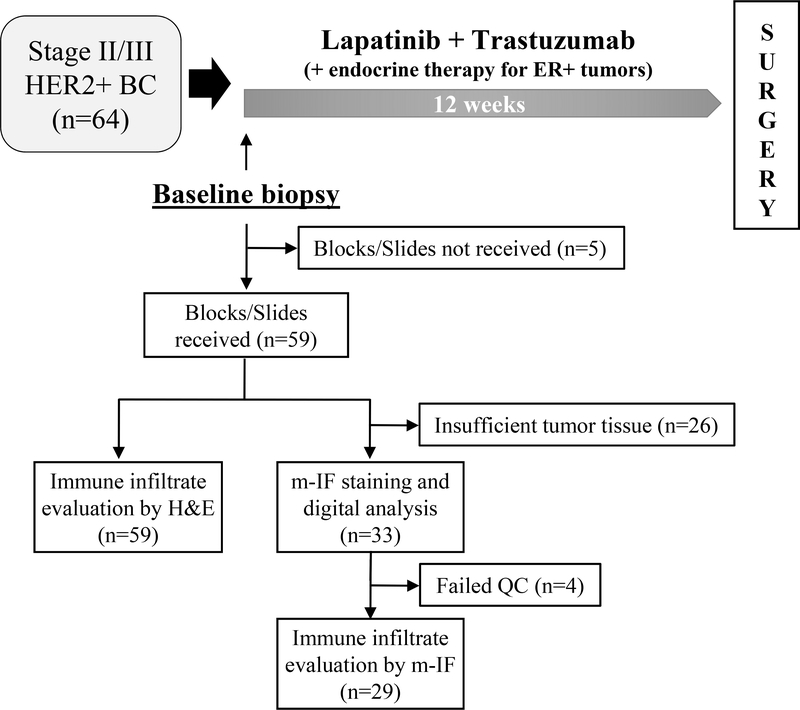
Figure 1. Evaluable tumor specimens for baseline immune infiltrate analysis in the TBCRC 006 trial.
Patients enrolled in the TBCRC 006 trial were treated for 12 weeks with neoadjuvant lapatinib plus trastuzumab (with endocrine therapy in ER-positive cases) followed by surgery. A total of 59 baseline tumor samples (pre-treatment) were analyzed for immune infiltration: all the samples by H&E staining and 33 also by multiplexed immunofluorescence. QC, quality control.
Tumor biopsies were collected before treatment (baseline) and placed in formalin for subsequent paraffin embedding (formalin-fixed paraffin embedded tissue, FFPE) or flash frozen on dry ice. Median tumor size was 6 cm (range, 1.5 to 30 cm). The overall pathological complete response (pCR) rate was 27% (36% in ER-negative tumors and 21% in ER-positive tumors).
Pathologic assessment
ER, PR, Ki67, and PTEN evaluation was performed by IHC; HER2 evaluation was by IHC and fluorescence in situ hybridization (FISH) assay, and PIK3CA mutational analysis was performed on baseline sample as previously described (1, 18, 19).
Standard assessment of TILs in H&E sections
Visual assessment of TILs was performed using full-face H&E slides by light microscopy according to the 2014 Guidelines from the International TILs Working Group (15). Briefly, all mononuclear cells, including lymphocytes and plasma cells and excluding granulocytes and other polymorphonuclear leukocytes, were identified as TILs. TILs within the borders of the invasive tumor stroma (s-TILs) were quantified as a percentage of occupied stromal areas (% s-TILs). TILs around ductal carcinoma in situ (DCIS), in necrotic areas, outside tumor border, and in normal breast tissue were not quantified. A threshold of 60% was used to distinguish LPBC from non-LPBC. Of the 64 patients enrolled on the TBCRC 006 trial, slides from baseline core biopsies were available for 59 (92.2%) patients.
Multispectral fluorescent immunohistochemistry
Multiplexed IF (m-IF) staining and multispectral image analysis (20) were also performed. Briefly, 4 μm thick formalin-fixed, paraffin-embedded (FFPE) slides were deparaffinized in a Leica autostainer using the following protocol: xylene 10 minutes, 100% ethanol 10 minutes, 95% ethanol 5 minutes, 70% ethanol 5 minutes, ddH2O briefly, 10% buffered formalin 10–20 minutes, ddH2O briefly. Antigen retrieval was performed using citrate buffer pH 6.0 and microwave treatment (45 seconds 100% power, 15 minutes 20% power). Slides were blocked with Antibody Diluent (Biogenex Technologies, Fremont, CA) for 10 minutes. Primary antibodies were diluted in Antibody Diluent and incubated for 30 minutes at room temperature. Primary antibodies (Suppl. Table 1) were subsequently removed by vacuum, and slides were washed in TBST. Supplementary Table 1 reports the list of primary antibodies used in this study. Anti-rabbit or anti-mouse IgG, HRP-linked antibodies (Life Technologies, Carlsbad, CA) were added drop-wise to slides. Slides were incubated for 10 minutes at room temperature and then washed in TBST. Tyramide (TSA)-conjugated fluorophores (Opal™, PerkinElmer, Inc., Hopkinton, MA) were added to slides at 1:50 dilution and incubated for 10 min at room temperature. TSA was vacuumed off and slides were washed in TBST. This process was repeated recursively for 6 antibodies. DAPI (Life Technologies, Carlsbad, CA) was diluted 1:500 in TBST and added to slides. Slides were incubated for 10 minutes at room temperature. Slides were then rinsed with ddH2O, cover slipped with VECTASHIELD Hard Mount (Vector Laboratories, Burlingame, CA), and stored at 4°C in a covered slide box. Slides were scanned using a Vectra automated multispectral microscope and images were analyzed using the inForm analysis software (PerkinElmer, Hopkinton, MA). For the majority of the biopsies the entire tumor and tumor microenvironment were included in the analyzed fields (5 fields 2×2, using 20x objective). For biopsies of larger size, 5 fields (2×2, using 20x objective) were selected for a significant sampling of the tumor and tumor microenvironment. A tissue segmentation algorithm was applied to define stromal and tumoral areas (20). Then, single or multiple IF-markers were used to localize, phenotype, and quantitate the density (number of positive cells per mm2) of specific cell types in both the stromal and tumoral areas (20).
Hierarchical clustering of immune cell markers
Distance metric equal to (1 – correlation) for standardized Log2 values (counts/mm2) from each case were used to conduct an unsupervised hierarchical clustering of the five m-IF immune markers. A centroid-linkage hierarchical clustering was performed using the dChip software (http://www.dchip.org/).
Statistical analysis
Spearman correlations, Fisher’s exact test, and Wilcoxon rank sum test were used to examine the associations between immune cells assessed by H&E or by m-IF and other tumor baseline biomarkers. The predictive value of s-TILs by H&E was tested by considering s-TILs as a continuous variable, and then according to the LPBC vs. non-LPBC categorical definition. Association of immune cell variables with pCR was determined by univariate and multivariable logistic regression analysis. Odd Ratios (OR) with a 95% confidence interval (95% CI) were estimated. In the multivariable model, the likelihood ratio test was used to evaluate the contribution of s-TILs, immune profiles by m-IF, and the clinicopathologic factors (age: ≤50 years, >50 years; categorical tumor size: ≤5 cm, >5 cm; positive nodes: yes, no). All statistical tests were two-sided and considered significant when P ≤ 0.05.
Results
Visual assessment of baseline s-TILs by H&E
Pretreatment s-TILs evaluation using H&E slides was conducted on 59 available samples out of the 64 patients who were evaluable for pCR (Figure 1). In our cohort, 12 patients (20%) exhibited an LPBC profile and 37 (80%) presented with a non-LPBC profile (Suppl. Figure 1). Patient demographic and clinical characteristics across baseline LPBC- and non-LPBC groups are reported in (Suppl. Table 2). The two groups did not significantly differ in age, race, ethnicity, menopausal status, performance status, tumor size, axillary lymph node involvement, expression of hormonal receptors (ER and PR) and deregulation of PI3K/PTEN pathway (Suppl. Table 2). We further investigated the correlations between s-TILs as continuous variable and levels of ER, PR, HER2 (HER2 H-score, and HER2/CEP17 ratio) and Ki67 (Suppl. Table 3). Our analysis showed a positive association of s-TILs with Ki67 (Spearman’s correlation = 0.304, P = 0.0241), but not with other tumor biomarkers (Suppl. Table 3).
We next evaluated the association of s-TILs with pCR. Levels of s-TILs considered either as a categorical or a continuous parameter were not significantly associated with pCR (high- vs. low-, P = 0.057; 10% increase OR = 1.22; 95% CI 0.97 – 1.53, P = 0.094). The pCR rate, however, was numerically higher in patients with LPBC compared to patients with non-LPBC (50% vs. 19%) was observed (Table 1). Similar results were found when we investigated the association of s-TILs with pCR in ER-positive or ER-negative subgroups (Suppl. Table 4).
Table 1.
Association between pathological complete response (pCR) and s-TILs
| pCR | Non-pCR | |||||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | Lower limit | Upper limit | Fisher’s Exact P | |
| Non-LPBC (n=47) | 9 | 19.1 | 38 | 80.9 | 1 | - | - | 0.0575 |
| LPBC (n=12) | 6 | 50 | 6 | 50 | 4.2 | 1.1 | 16.2 | |
Quantitative assessments of baseline immune cells by m-IF staining and digital analysis
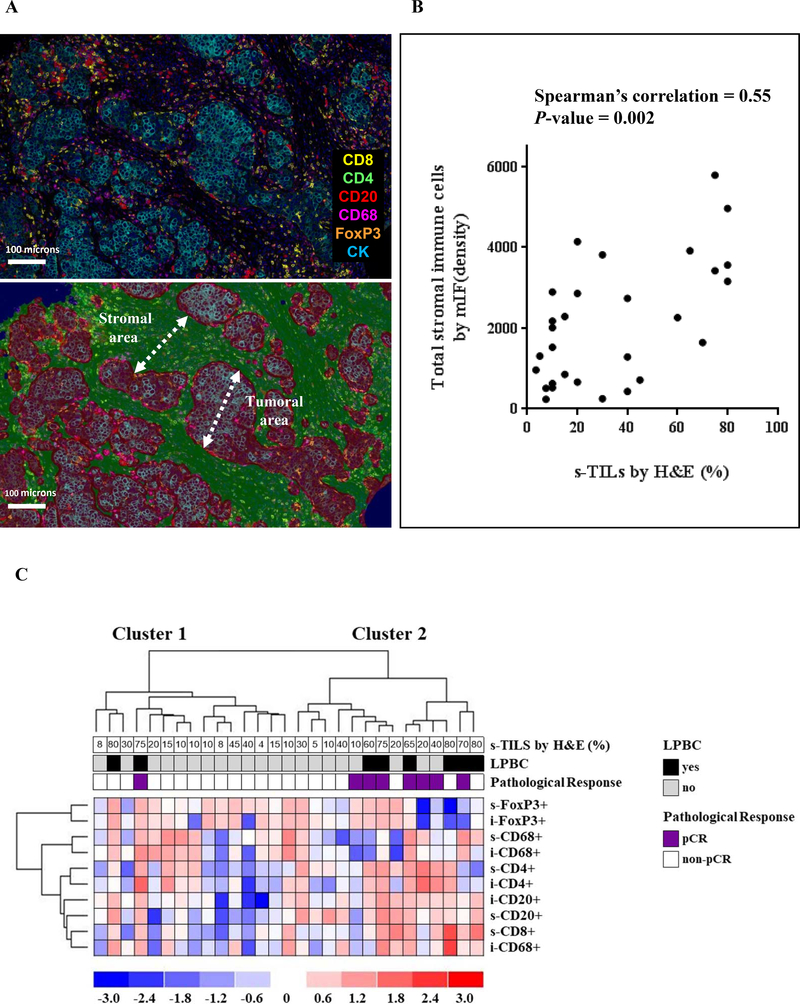
Next, we characterized and quantified various subtypes of stromal and intra-tumoral immune infiltrates [cytotoxic T cells (CD8+), helper T cells (CD4+/FoxP3-), regulatory T cells (FoxP3+), B cells (CD20+), and macrophages (CD68+)] using m-IF labeling and digital image analysis of 33 available tumor biopsies at baseline (Figure 1, 2A). In 29 out of 33 m-IF-stained samples, we successfully measured the density of s-TILs and i-TILs using the multispectral imaging data. One sample did not show the presence of tumor cells while three samples failed the m-IF staining quality control check. Importantly, baseline clinico-pathological characteristics did not significantly differ between groups of patients with (n = 29) or without (n = 30) m-IF data (Suppl. Table 5). Mean baseline values of s- and i-TILs density for the whole group are shown in Supplementary Table 6. The density of total s-TILs (total number of cells positive/mm2) determined by m-IF significantly and positively correlated with the percentage of s-TILs assessed on H&E-stained tissue sections from the same breast cancer specimens (r = 0.55, P = 0.002; Figure 2B). Furthermore, the densities of individual immune subtypes within stromal and intra-tumoral compartments were highly correlated (Supplementary Table 7). Infiltration of CD4+, CD8+, and CD20+ TILs showed strong positive correlations amongst one another in stromal as well as intra-tumoral areas (Suppl. Table 7). Finally, we also observed a significant correlation between levels of s-TILs assessed by H&E and s-CD20+, i-CD20+, s-CD8+, i-CD8+, and i-CD4+ cells (Suppl. Table 7).
Figure 2. Multiplexed immunofluorescence (m-IF) of tumor infiltrates of TBCRC 006 baseline specimens.
(A) The upper panel shows a composite image obtained using the mIF labeling and multispectral imaging. The m-IF stains included anti-CD8 (yellow), anti-CD4 (green), anti-CD20 (red), anti-CD68 (magenta), anti-FoxP3 (orange), and anti-CK (cyan); the bottom panel shows the stromal and tumoral areas defined using the inForm tissue segmentation algorithm. (B) Spearman’s correlation between total stromal TILS by mIF and s-TILs assessed by H&E. (C) Unsupervised hierarchical clustering analysis of pre-treatment TILs subpopulations.
Exploratory analysis of TILs as a predictor of response to chemotherapy-sparing neoadjuvant LT therapy in HER2+ breast cancer
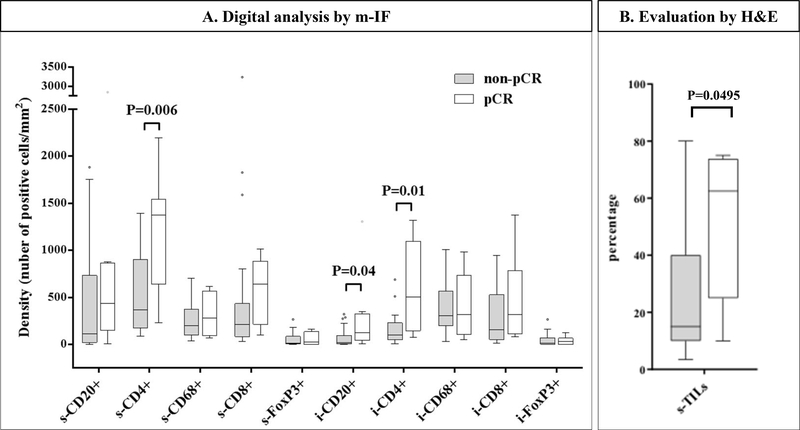
To investigate the immune subpopulation profiles and their relationship with response to dual anti-HER2 therapy, we performed an unsupervised hierarchical clustering using the five immune cell markers employed to characterize immune infiltrates. Clustering analysis generated two groups of tumors arbitrarily defined as Cluster 1 (n = 15, 51.7%) and Cluster 2 (n = 14, 48.3%) (Figure 2C). Cluster 2 group represented tumors with higher median levels of CD4+, CD20+, and CD8+ s-TILs and higher CD20+ i-TILs compared to Cluster 1 (Suppl. Table 8). No significant differences were found regarding clinico-pathological characteristics between the 2 cluster groups (Suppl. Table 9). A significant predictive value for pCR was demonstrated for the Cluster 2 profile in a univariate analysis (Odds Ratio: 14; 95% CI: 1.43 to 137.2, P = 0.0235; Table 2). Importantly, independent predictive value of Cluster 2 was also confirmed in a logistic regression multivariate analysis including age, tumor size, and axillary lymph node involvement (Table 2). In evaluating individual immune subsets, we found that patients who achieved pCR had higher levels of s-CD4+ (P = 0.006), i-CD4+ (P = 0.01), and i-CD20+ (P = 0.04) cells compared to patients who did not (Figure 3A). Conversely, s- or i- infiltration of CD8+, CD68+, FOXP3+, and s-CD20+ cells (Figure 3A) as well as three different TIL ratios (FOXP3+/CD4+, FOXP3+/CD8+, and CD8+/CD4+) did not correlate with treatment outcome (data not shown).
Table 2.
Univariate and multivariate analysis of correlation between immune infiltrate and pCR
| OR | Lower limit | Upper limit | P-value | ||
|---|---|---|---|---|---|
| Univariate model | |||||
| Cluster | 1 | 1 | - | - | |
| 2 | 14 | 1.43 | 137.3 | 0.0235 | |
| Multivariate model | |||||
| Cluster | 1 | 1 | - | - | |
| 2 | 19.073 | 1.221 | 298.035 | 0.0355 | |
| Age | >50 | 1 | - | - | |
| ≤50 | 2.561 | 0.265 | 24.73 | 0.4163 | |
| Tumor size | >5cm | 1 | - | - | |
| ≤5cm | 3.439 | 0.228 | 51.986 | 0.3727 | |
| Nodes | Y | 1 | - | - | |
| N | 1.523 | 0.142 | 16.349 | 0.7281 | |
Figure 3. Association between immune cell density and response to neoadjuvant trastuzumab plus lapatinib therapy.
Box plots of density of individual immune cell subpopulations in the stromal (s-) and intratumoral (i-) areas (A), and box plots of percentage of s-TILs assessed by H&E (B) in patients achieving a pathological complete response (pCR, white) versus residual disease (non-pCR, grey). P-values were not adjusted for multiple comparisons.
Within the subset of pre-treatment tumor samples analyzed by m-IF, the median level of s-TILs by H&E was higher in patients who achieved a pCR after neoadjuvant LT compared to those who did not (Figure 3B). Further, a significant association between s-TILs either as a continuous (P = 0.0371) or categorical (P = 0.0188) variable and pCR was observed in univariate analysis (Suppl. Table 10). In multivariable analyses, only the LPBC profile was found to be independently associated with pCR (Suppl. Table 10). Finally, using a likelihood ratio test, we investigated the added predictive information of cluster profiles to standard s-TILs and clinical prognostic factors. Our exploratory analysis revealed that cluster profiles added significant predictive information beyond that provided by conventional clinicopathologic variables and s-TILs as a continuous variable (P = 0.018). In contrast, the cluster profile did not add additional information to the model with s-TILs as categorical variable (P = 0.088).
Discussion
In the last few years, the tumor immune microenvironment has become an area of intense research in breast cancer (5, 6, 22). The tumor-host immune interaction may be particularly significant in determining the therapeutic effect of the monoclonal antibodies trastuzumab and/or pertuzumab, since their therapeutic efficacy depends in part on innate and adaptive immune-mediated mechanisms such as antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (22). This tumor-host immune interaction may be even more important in the context of LT therapy, which was used in our study. In fact, it has been suggested that the synergistic effect of lapatinib and trastuzumab may stem, at least partly, from an increased trastuzumab-mediated ADCC due to a lapatinib-induced accumulation of HER2 at the cell surface, as shown in preclinical models (22). Previous reports have shown that in patients with HER2+ breast cancer, high levels of TILs and the activation of immune pathways assessed by immune-related genes and gene signatures have been associated with more favorable prognosis in the adjuvant and metastatic settings as well as with increased rates of pCR upon neoadjuvant treatment (5, 6, 8, 9, 22–24). Most published data, however, stem from trials that studied regimens of anti-HER2 therapy in combination with chemotherapy. Although some of these trials included a chemotherapy alone control arm, the predictive value of tumor immune infiltrates inferred from such trials may not be reflective of the predictive role of immune infiltrates in the context of anti-HER2 treatment alone without the confounding effect of chemotherapy.
Recently, the predictive role of TILs has been evaluated in the PAMELA trial, where the chemo-free lapatinib plus trastuzumab (with endocrine therapy for ER-positive cases) regimen given for 18 weeks resulted in an overall pCR rate of 30% (3). In this study, high TILs at baseline significantly correlated with pCR in the univariate analysis, but the association between TILs and pCR did not maintain statistical significance in multivariate analysis corrected for intrinsic subtype, hormone receptor status, histological grade, and nodal status. Interestingly, TILs measured at day 15 of treatment and a combined score taking into account TILs and tumor cellularity (CelTIL) at day 15 of treatment were independently associated with pCR (12).
In our study, s-TILs were evaluated using full-face H&E slides, in accordance with the current recommended methodology. In line with the previous studies (13, 14), high levels of s-TILs were observed in 20% of patients and were not associated with deregulation of the PI3K pathway (due to PIK3CA mutations or loss/low PTEN protein levels). In contrast to previous reports, however, we did not find a significant association between increased s-TILs and ER-negative status (11, 13, 14), though we did notice a positive correlation of s-TILs with the Ki67 index, similar to the CherLob study (11). We further observed a numerically higher, but not statistically significant, pCR rate in patients with LPBC than in non-LPBC (50% vs 19%). Notably, in our cohort, no patients with levels of s-TILs lower than 10% achieved a pCR. Similar results have also been reported in the NeoALTTO and the NeoSphere trials, where patients with very low levels of TILs (s-TILs = <5%) showed the lowest pCR rates (13, 14).
The evaluation of TILs using an H&E-based method is semi-quantitative and provides limited information about the complexity of the tumor immune microenvironment, which may be crucial for a better understanding of the biology and clinical activity of the immune infiltrates. Several studies have investigated the predictive and/or prognostic value of specific immune cell markers, checkpoints, and gene expression signatures in breast cancer using different methodological approaches (5, 6, 14, 15, 25–27). A correlative analysis from the Cancer and Leukemia Group B (CALGB) 40601 has identified an immune-cell infiltrate signature (IgG signature) as an independent predictor of pCR (27). Similarly, in the GeparSixto trial, s-TILs as well as 12 immune-activating and immune-suppressive markers were found to be predictive of pCR in triple-negative and HER2+ breast cancer patients (26). Therefore, we attempted to further investigate the composition of the immune infiltrates by adapting an m-IF technology to quantify and appropriately classify the various immune cell subtypes present in the tumor (20). While our analysis has been conducted only in a subset of the patients, we have clearly demonstrated that this approach is feasible using FFPE tumor material. More importantly, the significant correlation observed between the classical visual assessment (15) of TILs and the automated digital image-based quantification of immune cells further validates this new analytical approach.
In this study, we used an m-IF panel of 5 phenotypic biomarkers which allowed us to simultaneously identify cytotoxic (CD8+) and helper (CD4+/Foxp3-) T-cells, T-reg cells (CD4+/FoxP3+), B-cells (CD20), and macrophages (CD68). Our profiling of the immune context resulted in identification of a group of patients (Cluster 2) characterized by high CD4, CD8, and CD20 infiltration, which was independently and significantly associated with pCR. Interestingly, evaluation of the individual immune subsets showed that high levels of CD4+ T-cells and CD20+ B-cells rather than CD8+ T-cells were more frequent in patients who achieved a pCR. Our results are in line with previous data on the predictive/prognostic values of TIL subpopulations in breast cancer. Several immunohistochemical studies reported increased CD8+ lymphocyte infiltration as a positive predictive and prognostic marker in breast cancer (28–30). Similarly, high levels of CD4+ T-cells, which play a key role in recruiting and modulating cytotoxic T-cells in antitumor immunity (31), have been associated with better survival (32, 33) and higher pCR rate (34). CD4+ TILs play a central role in recruiting and modulating B-cell and cytotoxic T-cell antitumor immunity. Notably, it has been shown that an adequate CD4+ T-cell activity is required for the CD8+ cytotoxic T-cells to fully function (31). Importantly, in the context of HER2+ disease, a recent study demonstrated that levels of IFNγ-secreting CD4+ T cells were higher in patients who achieved pCR than in those who did not (98% vs 33%) after neoadjuvant chemotherapy plus trastuzumab treatment (35).
Although not as well established as for T cells, a role for B cells in antitumor response has been suggested (36, 37). The presence of high stromal tumor-infiltrating B lymphocytes (CD20+ cells) assessed by IHC (34, 38) or by multiplexed quantitative immunofluorescence (25) has been linked to a favorable response to neoadjuvant chemotherapy in breast cancer patients. The expression of B-cell related genes and gene signatures has also been strongly associated with good prognosis in breast cancer (39, 40). In contrast, in two neoadjuvant trials of anti-HER2 treatment in combination with chemotherapy, B-cell signatures did not correlate with pCR (11, 27). In our quantitative m-IF analysis, higher levels of both stromal and intra-tumoral CD20+ B cells defined the Cluster 2 patients with higher pCR rates. Therefore, our data support a potential role for B cells in response to a dual anti-HER2 blockade in the absence of chemotherapy, which needs validation in future studies. Concordantly, patients who achieved pCR also showed numerically higher levels of stromal CD20+ cells compared to patients who did not, although, this difference did not reach statistical significance.
Our work presents some limitations. In our study, the lack of a statistically significant association of s-TILs with pCR could be ascribed to the small sample size in the TBCRC 006 trial or to a lack of biological association between TILs and response to dual HER2-targeted therapy (plus endocrine therapy for ER-positive cases) in the absence of chemotherapy. Other limitations include the limited number of immune markers used for the characterization of the immune infiltrates and the lack of data to address its role in affecting long-term outcomes. Finally, despite intriguing results from the clustering analysis, we recognize that larger data sets are required in order to develop an m-IF-based predictor that could prospectively assign a molecular immunological subtype to each HER2+ breast cancer case.
In conclusion, our study presents a strong rationale for conducting quantitative assessment of the immune cell infiltrates using m-IF to correlate the individual immune cell profile with response. We have identified an immune profile in HER2+ breast cancer patients that is an independent predictor of pCR. Future and ongoing studies with larger patient populations combined with external validation of the predictive value of the immune subsets will further elucidate the repertoire of immune infiltrates that provide predictive and/or prognostic information to guide the treatment of patients with HER2+ breast cancer.
Supplementary Material
Translational Relevance.
While several clinical trials have suggested that combination of anti-HER2 agents without chemotherapy may be an effective treatment approach for a subset of patients with HER2+ breast cancer, there is still a lack of appropriate biomarkers to accurately select these patients. Additionally, while the distribution of tumor-infiltrating lymphocytes has been proven to predict therapeutic response and long-term outcomes in HER2+ breast cancer, novel quantitative methods to more precisely assess their density and distribution are also lacking. Our study demonstrates that multispectral imaging of pre-treatment tumor specimens by multiplexed immunofluorescence is feasible and effective in quantifying different subtypes of lymphocytic infiltrates, and may allow for the identification of an immune infiltrate profile characterized by high CD4+, CD8+, and CD20+ cells, which is independently and significantly associated with pCR. Overall, our findings suggest that this technique bears the potential as a quantitative and qualitative assay to precisely delineate the immune infiltrates in tumor specimens and aid in predictive biomarker discovery.
Acknowledgements
Financial support:
Research reported in this publication was supported in part by the NIH SPORE Grants (P50 CA058183 and CA186784 to RS, CKO, and MFR); Cancer Center Grants (P30 CA125123, P30 CA008748); the Breast Cancer Research Foundation (to RS, CKO, and JSR-F; no grant number applies); research grant from the Breast Cancer Research Foundation BCRF-17-143 (to RS and CKO); the Cancer Prevention & Research Institute of Texas CPRIT RP 140102 and the Conquer Cancer Foundation—Gianni Bonadonna Breast Cancer Research Fellowship (to CDA); Instituto de Salud Carlos III - PI16/00904 (to AP); Department of Defense grants W81XWH-17-1-0579 (to MFR) and W81XWH-17-1-0580 (to RS); and the Translational Breast Cancer Research Consortium (TBCRC). We are grateful for the funding support from the TBCRC, the AVON Foundation, the Breast Cancer Research Foundation, and Susan G. Komen
Conflicts of interest.
Carmine De Angelis: nothing to disclose, Chandandeep Nagi: nothing to disclose, Cliff Hoyt: employee and stock holder of Akoya Biosciences, Liu Linying: employee of Akoya Biosciences, Kristin Roman: employee of Akoya Biosciences, Chichung Wang: employee of Akoya Biosciences, Yi Zheng: employee of Akoya Biosciences, Jamunarani Veeraraghavan: nothing to disclose, Vidyalakshmi Sethunath: nothing to disclose, Paolo Nuciforo: Compensated consulting role for Bayer and MSD; travel expenses from Novartis, Tao Wang: nothing to disclose, Anna Tsimelzon: nothing to disclose, Sufeng Mao: nothing to disclose, Susan G. Hilsenbeck: nothing to disclose, Meghana V. Trivedi: nothing to disclose, Maria Letizia Cataldo: nothing to disclose, Anne C. Pavlick: nothing to disclose, Antonio C. Wolff: Research grant from Genentech (to Institution), Britta Weigelt: nothing to disclose, Jorge S. Reis-Filho: Paid consultant fees by Goldman Sachs Merchant Banking; Member of the scientific advisory board with paid honoraria of Paige.AI, Volition Rx, Roche Tissue Diagnostics, Ventana, InVicro, Genentech, and Roche, Aleix Prat: Research funding from Nanostring Technologies; Advisory boards for Nanostring Technologies; Novartis; and Roche, Carolina Gutierrez: Research grant from Ventana, C. Kent Osborne: Research funding from AstraZeneca and GlaxoSmithKline; Advisory boards for Tolmar Pharmaceuticals, Genentech, and AstraZeneca; DMC for Eli Lilly; stockholder of GeneTex, Mothaffar F. Rimawi: Research grant from GlaxoSmithKline (to Institution), Consulting with Genentech, Novartis, Daiichi, and Macrogenics, Rachel Schiff: Research funding from AstraZeneca, GlaxoSmithKline, Gilead Sciences, and PUMA Biotechnology; Consulting/advisory committee member for Macrogenics, and Eli Lilly.
List of abbreviations
- ASCO
American Society of Clinical Oncology
- BC
breast cancer
- CAP
College of American Pathologists
- ER
estrogen receptor
- FISH
fluorescence in situ hybridization
- FFPE
formalin-fixed paraffin embedded tissue
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- i-
intratumoural
- LPBC
lymphocyte-predominant BC
- LT
lapatinib plus trastuzumab regimen
- m-IF
multiplexed immunofluorescence
- pCR
pathologic complete response
- PR
progesterone receptor
- QC
quality control
- S-
stromal
- TILs
tumor infiltrating lymphocytes
BIBLIOGRAPHY
- 1.Rimawi MF, Mayer IA, Forero A, Nanda R, Goetz MP, Rodriguez AA, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol. 2013;31(14):1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rimawi MF, Niravath PA, Wang T, Rexer BN, Forero A, Wolff AC, et al. TBCRC023: A randomized multicenter phase II neoadjuvant trial of lapatinib plus trastuzumab, with endocrine therapy and without chemotherapy, for 12 vs. 24 weeks in patients with HER2 overexpressing breast cancer. The Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2014; San Antonio: AACR Cancer Res. [Google Scholar]
- 3.Llombart-Cussac A, Cortes J, Pare L, Galvan P, Bermejo B, Martinez N, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017;18(4):545–54. [DOI] [PubMed] [Google Scholar]
- 4.Veeraraghavan J, De Angelis C, Reis-Filho JS, Pascual T, Prat A, Rimawi MF, et al. De-escalation of treatment in HER2-positive breast cancer: Determinants of response and mechanisms of resistance. Breast. 2017;34 Suppl 1:S19–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nature Reviews Clinical Oncology. 2016;13(4):228–41. [DOI] [PubMed] [Google Scholar]
- 6.Luen SJ, Savas P, Fox SB, Salgado R, Loi S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology. 2017;49(2):141–55. [DOI] [PubMed] [Google Scholar]
- 7.Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, et al. Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res. 2016;22(23):5747–54. [DOI] [PubMed] [Google Scholar]
- 8.Ignatiadis M, Van den Eynden G, Roberto S, Fornili M, Bareche Y, Desmedt C, et al. Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: a TRYPHAENA substudy. J Natl Cancer Inst. 2019;111(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieci MV, Conte P, Bisagni G, Brandes AA, Frassoldati A, Cavanna L, et al. Association of tumor-infiltrating lymphocytes with distant disease-free survival in the ShortHER randomized adjuvant trial for patients with early HER2+ breast cancer. Ann Oncol. 2019;30(3):418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchini G, Kiermaier A, Bianchi GV, Im YH, Pienkowski T, Liu MC, et al. Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Research. 2017;19(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieci MV, Prat A, Tagliafico E, Pare L, Ficarra G, Bisagni G, et al. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Ann Oncol. 2016;27(10):1867–73. [DOI] [PubMed] [Google Scholar]
- 12.Nuciforo P, Pascual T, Cortes J, Llombart-Cussac A, Fasani R, Pare L, et al. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol. 2018;29(1):170–7. [DOI] [PubMed] [Google Scholar]
- 13.Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchini G, Pusztai L, Pienkowski T, Im YH, Bianchi GV, Tseng LM, et al. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann Oncol. 2015;26(12):2429–36. [DOI] [PubMed] [Google Scholar]
- 15.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45. [DOI] [PubMed] [Google Scholar]
- 17.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134(7):e48–72. [DOI] [PubMed] [Google Scholar]
- 18.Rimawi MF, De Angelis C, Contreras A, Pareja F, Geyer FC, Burke KA, et al. Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer. Breast Cancer Res Treat. 2018;167(3):731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veeraraghavan J, De Angelis C, Mao R, Wang T, Herrera S, Pavlick AC, et al. A combinatorial biomarker predicts pathologic complete response to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2+ breast cancer. Ann Oncol. 2019; 30(6): 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. [DOI] [PubMed] [Google Scholar]
- 21.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15(2):e58–68. [DOI] [PubMed] [Google Scholar]
- 23.Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18(1):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez EA, Thompson EA, Ballman KV, Anderson SK, Asmann YW, Kalari KR, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J Clin Oncol. 2015;33(7):701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2014;20(23):5995–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–91. [DOI] [PubMed] [Google Scholar]
- 27.Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;34(6):542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Research. 2012;14(2):R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25(8):1536–43. [DOI] [PubMed] [Google Scholar]
- 30.Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. British Journal of Cancer. 2013;109(10):2705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Research. 2010;70(21):8368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung YR, Kim HJ, Jang MH, Park SY. Prognostic value of tumor infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res Treat. 2017;161(3):409–20. [DOI] [PubMed] [Google Scholar]
- 33.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123(7):2873–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Martinez E, Gil GL, Benito AC, Gonzalez-Billalabeitia E, Conesa MA, Garcia Garcia T, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast cancer research : BCR. 2014;16(6):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta J, Berk E, Xu S, Fitzpatrick E, Rosemblit C, Lowenfeld L, et al. Anti-HER2 CD4(+) T-helper type 1 response is a novel immune correlate to pathologic response following neoadjuvant therapy in HER2-positive breast cancer. Breast Cancer Research. 2015;17:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–82. [DOI] [PubMed] [Google Scholar]
- 37.Tsou P, Katayama H, Ostrin EJ, Hanash SM. The emerging role of B cells in tumor immunity. Cancer Research. 2016;76(19):5597–601. [DOI] [PubMed] [Google Scholar]
- 38.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–13. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Research. 2008;68(13):5405–13. [DOI] [PubMed] [Google Scholar]
- 40.Iglesia MD, Vincent BG, Parker JS, Hoadley KA, Carey LA, Perou CM, et al. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res. 2014;20(14):3818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.