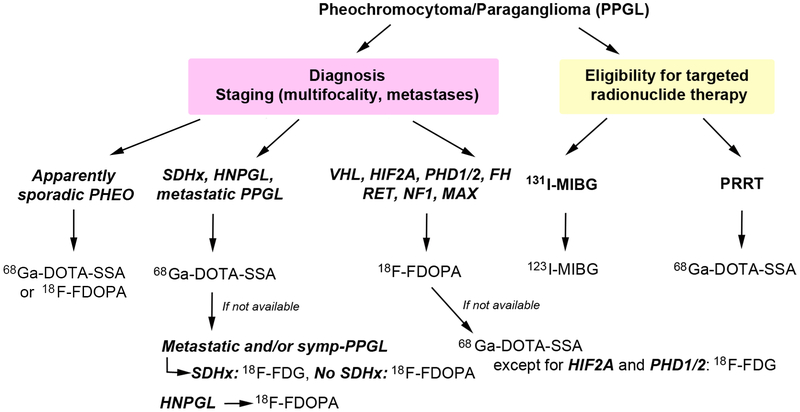
Figure 2. Proposed clinical algorithm for nuclear imaging investigations of pheochromocytoma/paraganglioma patients according to genotype.
The algorithm has been proposed for patients with suspected or confirmed PPGL in order to help towards the appropriate use of PET radiopharmaceuticals across PPGL subtypes. It may suffer from potential limitation of reported small number of cases. The optimal follow-up algorithm for non-proband SDHx-associated PPGLs remains to be determined but mostly relies on annual biochemical screening and MRI at regular intervals.
CT/MRI: anatomic imaging with computed tomography and/or magnetic resonance imaging; 18F-FDG: 18F-fluorodeoxyglucose; 18F-FDOPA: 18F-fluorodihydroxyphenylalanine; FH: fumarate hydratase gene, 68Ga-DOTA-SSA: gallium-68 (68Ga)-labeled somatostatin receptor analogs; HNPGL: head and neck paraganglioma; HIF2A: hypoxia-inducible factor 2 alpha, MAX: germline mutation of MYC-associated factor X gene; NF1: neurofibromatosis 1 gene; N.A.: not available; PHEO: pheochromocytoma; PPGL: pheochromocytoma/paraganglioma; PET/CT: positron emission tomography-computed tomography; PHD1/2: prolyl hydroxylase 1 and 2 gene; RET: rearranged during transfection gene; SDHB: germline mutation of succinate dehydrogenase subunit B gene; SSA: somatostatin analog; SDHx: germline mutation in one of the succinate dehydrogenase complex genes; symp-PPGL: sympathetic PPGL; PRRT: peptide receptor radionuclide therapy.