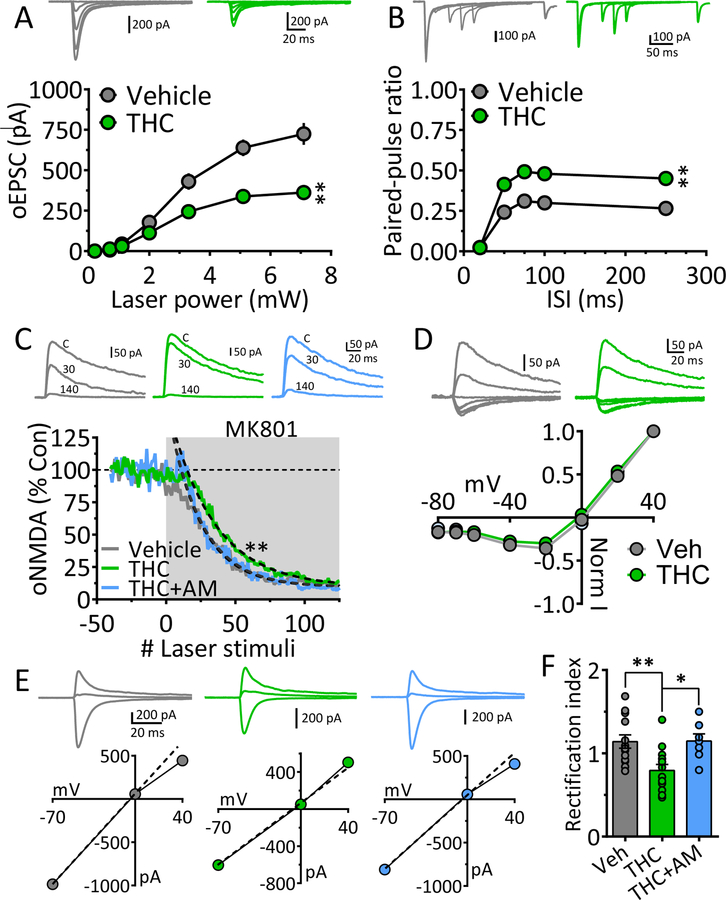
Figure 2. Mechanisms underlying weakened mPFC glutamatergic input to NAcs following chronic Δ9-THC.
A. Input-output (I-O) curves showing relationship between laser power, activating ChR2 in mPFC axons projecting to NAcs, and oEPSC amplitudes after chronic vehicle or Δ9-THC injections. Representative mean oEPSCs are shown at 5 laser intensities in neurons from vehicle (gray traces) and Δ9-THC-injected (green traces) rats. The strength of mPFC glutamate input to NAcs neurons was significantly smaller following chronic Δ9-THC (** = F18,342 = 9.2, p < 0.0001, intensity x treatment interaction, two-way repeated measures ANOVA).B. Mean paired-pulse ratios (PPR) of mPFC-evoked oEPSCs at different inter-stimulus (laser pulse) intervals (ISI) in NAc neurons from chronic Δ9-THC- and vehicle injected rats. Mean representative traces from individual cells in both groups are shown above. The PPR was significantly increased by chronic Δ9-THC treatment (** = F12,208 = 3.9, p < 0.0001, ISI x treatment interaction, two-way repeated measures ANOVA). Note that paired oEPSCs overlapped at the 20 ms ISI, thereby yielding a ratio = 0. C. The rate of the progressive, activity-dependent, block of ChR2-evoked NMDA receptor oEPSCs by MK801, is significantly slower following chronic Δ9-THC in the mPFC → NAcs pathway (time-constant of oNMDA current reduction in MK801: chronic vehicle = 23.4 stimuli, 95% confidence interval = 22.2 – 24.5 stimuli, n = 8, gray; chronic Δ9-THC = 32.5 stimuli, 95% confidence interval = 30.9 – 34.2 stimuli, n = 8, green), and this was prevented by co-injection with the cannabinoid antagonist AM251 (chronic Δ9-THC + AM251 = 24.0 stimuli, 95% confidence interval = 22.8 – 25.4 stimuli, n = 4, blue). Exponential decay time constants (τ) were obtained by curve fitting (dashed lines). Representative oNMDA waveforms from each group obtained before MK801 application (control, C), and at 30 and 140 stimuli are shown above. Note the smaller reduction of the oNMDA current at 30 stimuli in the cell from the chronic Δ9-THC treated animal compared to the other groups. Recordings were performed in the presence of DNQX, a kainate/AMPA receptor antagonist, and picrotoxin, a GABAA Cl− channel blocker. D. Lack of chronic Δ9-THC effect on the voltage-dependence of oNMDA currents. Representative mean traces obtained from chronic vehicle (gray) and Δ9-THC-treated (green) cells are shown above. E. Chronic Δ9-THC causes a reduction in inward rectification of ChR2-evoked AMPA receptor-mediated synaptic currents at mPFC → NAcs synapses. Shown are representative current-voltage (I-V) relationships and mean waveforms of AMPA oEPSCs evoked by activation of ChR2 in the mPFC → NAcs pathway at 3 different membrane voltages (Vm = −70, 0, and +40) from each group (chronic vehicle, gray, chronic Δ9-THC, green, and chronic Δ9-THC + AM251, blue). A hypothetical slope = 1.0 is indicated by a dashed line. F. Mean rectification index (RI = absolute AMPA current measured at −70 mV divided by that measured at +40 mV) for all cells in each group. The cells from chronic Δ9-THC-treated animals showed a significant reduction in rectification index compared to chronic vehicle-, or chronic Δ9-THC + AM251-treated rats (F3,44 = 5.6, p < 0.001, one-way ANOVA, ** = p < 0.001, * = p < 0.05, Holm-Sidak post-hoc comparison, respectively). Number of neurons/number of rats: A. Veh: 16/11, THC: 18/ 6 B. Veh: 12/11, THC: 15/6. C. Veh: 8/6, THC: 8/5, THC+AM: 4/4. D. Veh: 9/6, THC: 7/5. F. Veh: 13/6, THC: 13/5, THC+AM: 7/4.