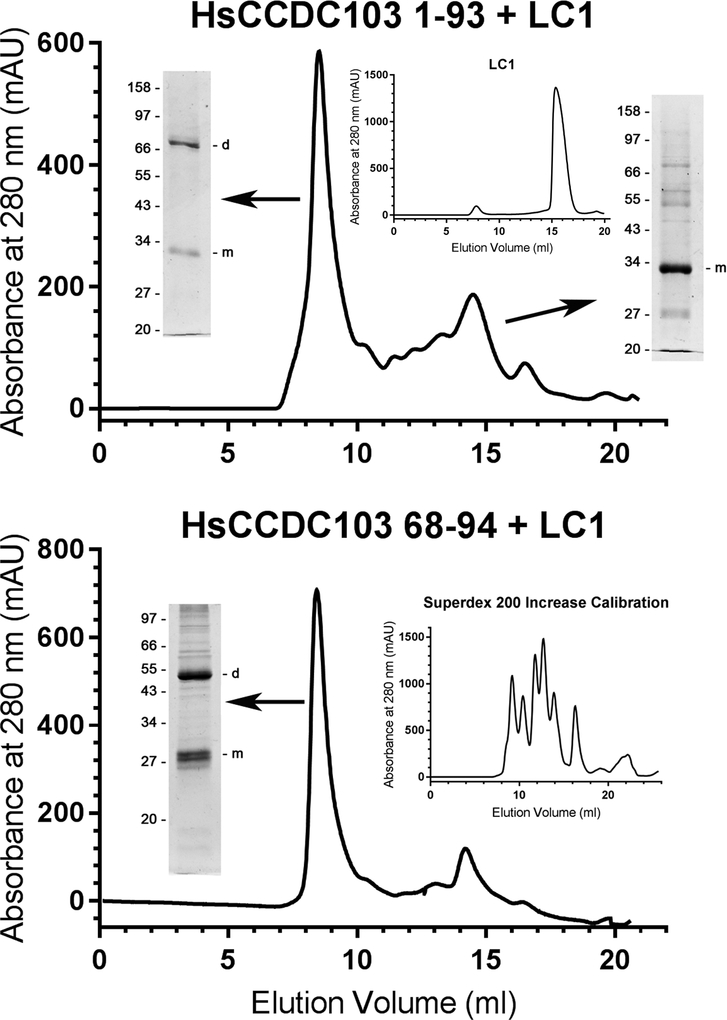
Fig. 2. The Inherently Disordered Region of HsCCDC103 Mediates Dimer Formation.
HsCCDC103 regions 1–93 and 68–94 were fused to the N-terminus of the Chlamydomonas LC1 dynein light chain which on its own behaves solely as a monomer (upper panel inset) (Wu et al., 2000; King and Patel-King, 2015). The fusion proteins were fractionated on a Superdex 200 Increase gel filtration column and samples from the peaks subject to SDS-PAGE. Both proteins gave high molecular mass peaks that were capable of forming detergent/heat resistant dimers. Previously, the N-terminal coiled coil was found to behave as a monomer (King and Patel-King, 2015). Thus, the 68–94 disordered region is sufficient to explain the unusual dimerization properties of HsCCDC103.