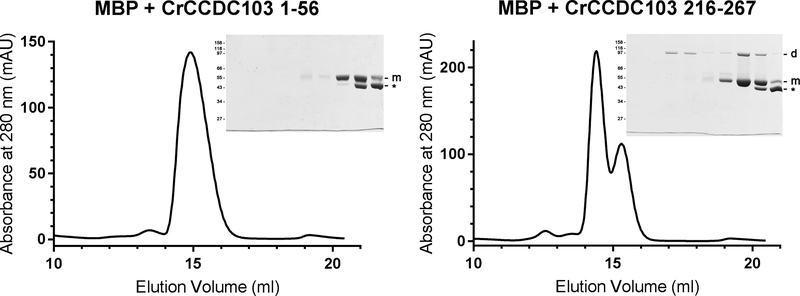
Fig. 4. CrCCDC103 Contains a C-terminal Dimerization Domain.
Two segments of CrCCDC103 encoding the terminal domain residues 1–56 and 216–267 were fused to MBP and fractionated by gel filtration. The 1–56 fusion protein migrated as a single monomer peak. SDS-PAGE revealed the MBP-CrCCDC103 monomer band and also a proteolytic fragment (indicated by *). In contrast, the C-terminal CrCCDC103 fusion yielded three peaks. SDS-PAGE analysis revealed that the largest consisted only of a fusion protein dimer; the second contained both dimer and monomer and was clearly resolved from the third peak that was almost completely formed from the proteolytic fragment alone. Thus, this C-terminal region of CrCCDC103 is capable of dimer formation.