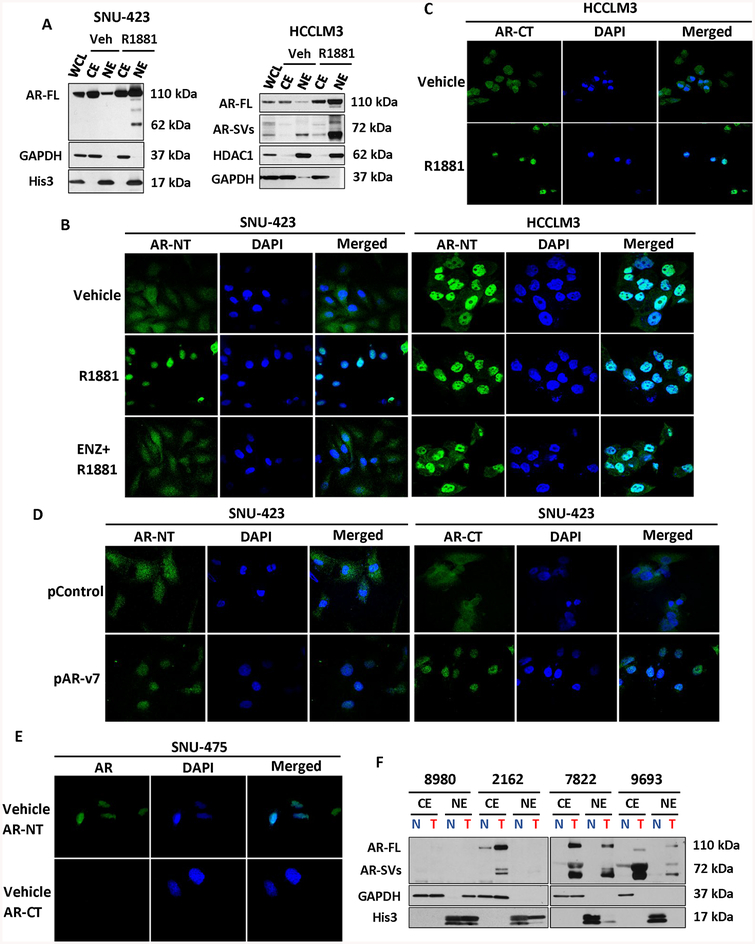
Figure 2. Androgen receptor splice variants are predominately nuclear and ligand-independent.
(a) Western blot of AR expression in SNU-423 (left) and HCCLM3 (right) cytoplasmic or nuclear fractions using an N-terminal targeting AR anti-body. Whole cell lysate (WCL), cytoplasmic extract (CE) and nuclear extract (NE) fractions were assayed after vehicle or 1 nM R1881 treatment for 24hours. In vehicle treated SNU-423 cells, the AR is mainly cytoplasmic but becomes predominantly nuclear following treatment with R1881. In contrast with SNU-423 cells, nuclear localized AR-SVs can be detected in untreated HCCLM3 cells. Following treatment with R1881, the expression of all nuclear localized AR species increases. GAPDH and Histone 3 or HDAC1 serve as cytoplasmic and nuclear controls, respectively. (b) Immunofluorescence analysis of AR in HCCLM3 performed using an N-terminal AR antibody (AR-NT, green) with DAPI nuclear counter stain visualized by confocal microscopy (60×). SNU-423 (left) were treated with either vehicle, 1 nM R1881, or androgen antagonist 10 μM enzalutamide (ENZ) with 1 nM R1881 for 24 hours. Matching nuclear fractionation in Figure 2A, AR stain was primarily diffuse and cytoplasmic in untreated SNU-423 but became nuclear following treatment with R1881. HCCLM3 (right) were similarly treated but revealed intense nuclear staining in the absence of androgen. R1881 treatment reduced the minimal cytoplasmic staining that was apparent in untreated cells but co-treatment with ENZ resulted in considerable residual nuclear localized AR. (c) Immunofluorescence analysis of AR in HCCLM3 with a C-terminal AR (AR-CT, green) antibody. In contrast with N-terminal staining in Figure 2B, C-terminal reactive AR is primarily cytoplasmic in the absence of the ligand but becomes nuclear localized when cells were treated with R1881 for 24 hours. (d) Immunofluorescence analysis of SNU-423 cells was performed after transfection of expression vectors encoding AR-v7 (pAR-v7), or plasmid control (pControl). An N-terminal AR monoclonal antibody was used to detect AR localization (green) as in Figure 2. B. Consistent with Figure 2B, AR localization as determined by AR-NT or AR-CT is predominantly cytoplasmic in untreated control plasmid transfected cells. Whereas transfection with pAR-v7, resulted in strong, predominantly nuclear staining with both AR-NT and AR-CT.(e) Immunofluorescence analysis of SNU-475 cells, consistent with Figure 1H–I, AR localization as determined by AR-NT is predominantly nuclear. Whereas AR was undetectable by AR-CT antibody. (f) CE and NE fractions of four representative, primary HCC samples analyzed for AR expression using an N-terminal reactive AR antibody. Tumor (T) AR expression is greater than patient matched, adjacent non-tumor (N) samples and multiple patients demonstrate expression of nuclear localized low molecular weight AR species. For Immunofluorescence experiments, AR localization was analyzed using the Olympus FluoView 4.2 program on Olympus FV 1000 spectral confocal microscope (panels B-D). DAPI staining (blue) indicates nuclei. All experiments were carried out in triplicate with representative fields presented.