FIG 4.
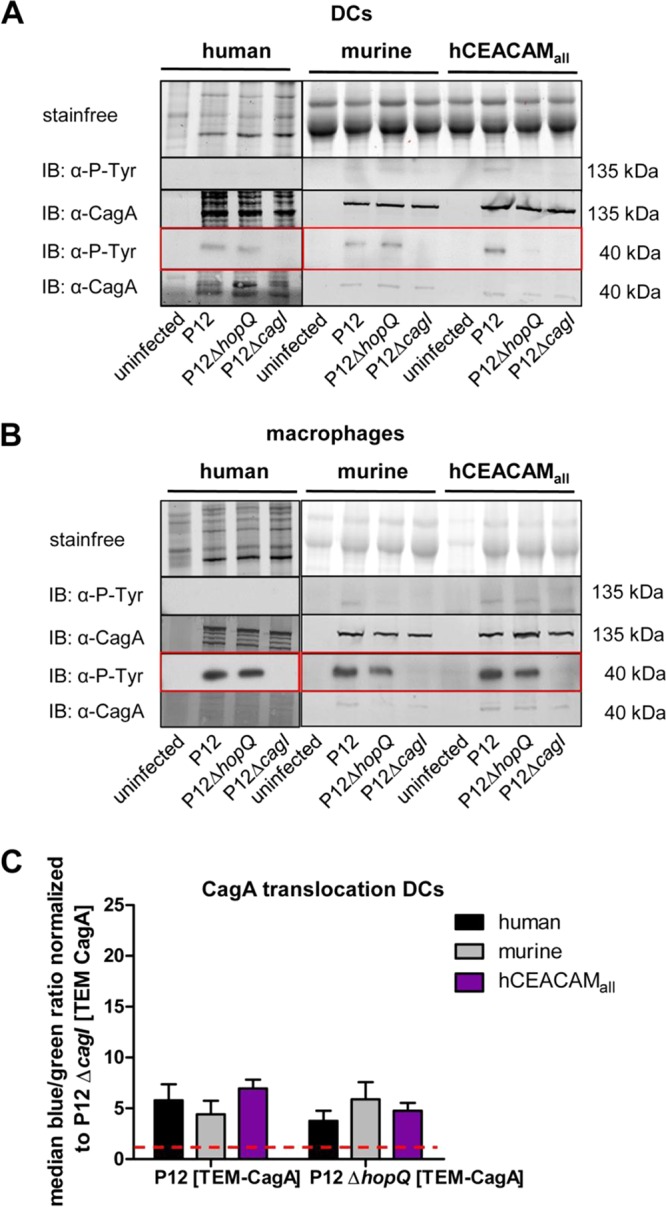
CagA translocation and its activation by tyrosine phosphorylation in human, mouse, and CEACAM-humanized DCs and macrophages is independent of the HopQ-CEACAM interaction. Monocytes isolated from human blood or bone marrow of wt and CEACAM-humanized mice were differentiated to DCs (A) and macrophages (B) and infected with strain P12, strain P12ΔhopQ, or the P12ΔcagI strain for 3 h at an MOI of 60. Translocation of CagA was determined by detecting tyrosine-phosphorylated CagA (α-P-Tyr) with antibody PY99 or CagA with antibody AK299 in the immunoblot. The Stain-Free method was used as a loading control for immunoblotting experiments (49). Full-length tyrosine-phosphorylated or nonphosphorylated forms of CagA (∼135 kDa) or the processed C-terminal fragment (∼40 kDa) (see red box) are shown for all blots. (C) Quantitative evaluation of CagA translocation into human, murine, and CEACAM-humanized DCs by the TEM assay. DCs were infected with H. pylori P12[TEM-CagA] or the P12ΔhopQ[TEM-CagA] strain and normalized against the translocation-deficient P12ΔcagI[TEM-CagA] deletion mutant at an MOI of 60 (red dotted line) (n ≥ 3). Data were assessed using two-way ANOVA and the Bonferroni post hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
