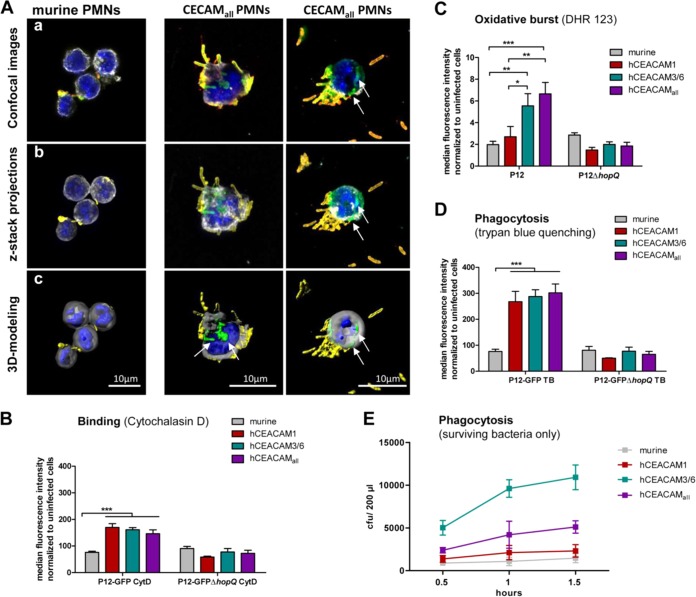
FIG 6.
Human CEACAMs on mouse neutrophils promote H. pylori binding, oxidative burst generation, and bacterial internalization and support H. pylori intracellular survival. (Aa) Confocal laser scanning images showing murine PMNs (left) and hCEACAMall PMNs (right) infected with H. pylori after an extracellular/intracellular staining procedure, showing extracellular H. pylori (yellow/orange) and intracellular H. pylori (green). The PMN nucleus is stained by DAPI (blue), and the borders of the cell are labeled with anti-WGA-Alexa647 (gray) (see Materials and Methods for further details). (Ab) Confocal images taken with z-stacks and the projections reconstructed in a three-dimensional cell volume. (Ac) Imaging projections modeled using the Imaris software contour surface tool (see also Video S1). (B) CEACAM-humanized but not wt mouse neutrophils efficiently bind H. pylori in a HopQ-dependent manner, as determined by flow cytometry of cells treated with the actin polymerization inhibitor cytochalasin D to prevent phagocytosis (MOI of 25; 1 h). (C) Mouse wt or CEACAM-humanized neutrophils were infected with H. pylori P12, and oxidative burst responses were analyzed using the fluorescent probe dihydrorhodamine 123 (DHR 123) as described in Materials and Methods. (D) To quantify phagocytosed, intracellular H. pylori, neutrophils were infected with H. pylori P12-GFP or P12ΔhopQ-GFP (MOI of 25; 1 h), and the fluorescence of surface-bound extracellular bacteria was quenched with trypan blue. (E) Bacterial survival in neutrophils was evaluated as CFU present in PMN lysates after 1 h of infection after killing of extracellular bacteria and cell perforation (n = 3). Data were assessed using two-way ANOVA and the Bonferroni post hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.