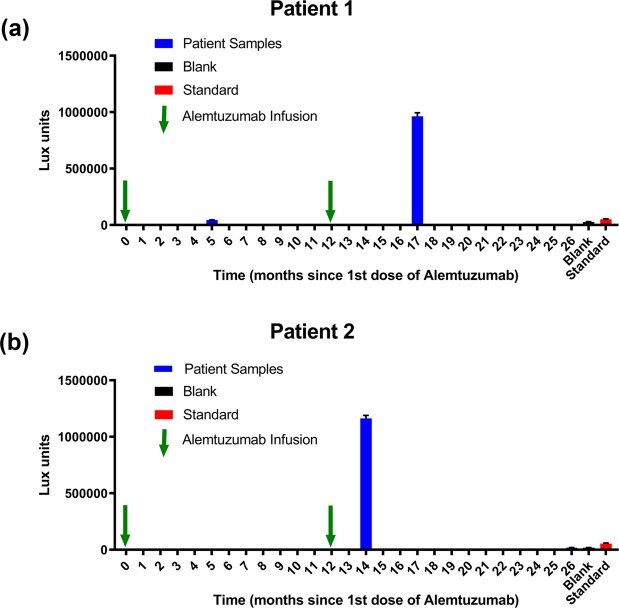
Figure 3.
Detection of ADA in serum samples from patients treated with alemtuzumab. (a) In patient 1, initially dosed with alemtuzumab at month 0 and a 1st serum sample taken at month 5, then dosed at month 12 and a 2nd serum sample taken at month 17. The control blank and the 50 µg/mL ADA positive included as reference points. Luciferase activity above the limit of detection were observed at both time points. In patient 2, (b) dosed with alemtuzumab at month 0 and then at month 12 prior to the 1st serum sample at month 14 and a 2nd serum sample taken at month 26. The control blank and the 50 µg/mL ADA positive included as reference points. Luciferase activity above the limit of detection was observed at 2 months after the 2nd infusion which then decreased to below the LoD at month 26.