Abstract
Background
This is the second update of a review originally published in 2017. Starting with one drug and with a combination of two drugs are strategies suggested in clinical guidelines as initial treatment for hypertension. The recommendations are not based on evidence about clinically relevant outcomes. Some antihypertensive combinations have been shown to be harmful. The actual harm‐to‐benefit balance of each strategy is unknown.
Objectives
To determine if there are differences in clinical outcomes between monotherapy and combination therapy as initial treatment for primary hypertension.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomised controlled trials up to March 2021: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (from 1946), and Embase (from 1974). The World Health Organization International Clinical Trials Registry Platform and the US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) were searched for ongoing trials. We also contacted authors of relevant papers regarding further published and unpublished work. We used no language restrictions. We also searched clinical studies repositories of pharmaceutical companies, reviews of combination drugs on the US Food and Drug Administration and European Medicines Agency websites, and lists of references in reviews and clinical practice guidelines.
Selection criteria
We included randomised, double‐blind trials with at least 12 months' follow‐up in adults with primary hypertension (systolic blood pressure/diastolic blood pressure 140/90 mmHg or higher, or 130/80 mmHg or higher if participants had diabetes), which compared a combination of two first‐line antihypertensive drugs with monotherapy as initial treatment. Trials had to include at least 50 participants per group and report mortality, cardiovascular mortality, cardiovascular events, or serious adverse events.
Data collection and analysis
Two review authors independently selected trials for inclusion, evaluated the risks of bias, and performed data entry. The primary outcomes were mortality, serious adverse events, cardiovascular events, and cardiovascular mortality. Secondary outcomes were withdrawals due to drug‐related adverse effects, reaching blood pressure control (as defined in each trial), and blood pressure change from baseline. Analyses were based on the intention‐to‐treat principle. We summarised data on dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). We used GRADE to assess the quality of the evidence.
Main results
We found no new trials for this update. The three original trials in the main comparison (monotherapy: 335 participants; combination therapy: 233 participants) included outpatients, mostly European and white people. Two trials only included people with type 2 diabetes; the remaining trial excluded people treated with diabetes, hypocholesterolaemia, or cardiovascular drugs. The follow‐up was 12 months in two trials and 36 months in one trial. The following treatments were compared: perindopril + indapamide versus enalapril; perindopril + indapamide versus atenolol; and verapamil + trandolapril versus verapamil or trandolapril.
Our 2019 update included one new study in which a subgroup of participants met our inclusion criteria. As none of the four included studies focused solely on people initiating antihypertensive treatment, we asked investigators for data for this subgroup. One study (PREVER‐treatment 2016) used a combination of thiazide‐type diuretic/potassium‐sparing diuretic: chlorthalidone + amiloride compared to losartan. As the amiloride is not indicated in monotherapy, we analysed this study separately.
It is very uncertain whether combination therapy versus monotherapy reduces total mortality (RR 1.35, 95% CI 0.08 to 21.72), cardiovascular mortality (zero events reported), cardiovascular events (RR 0.98, 95% CI 0.22 to 4.41), serious adverse events (RR 0.77, 95% CI 0.31 to 1.92), or withdrawals due to adverse effects (RR 0.85, 95% CI 0.53 to 1.35); all outcomes had 568 participants, and we rated the evidence as of very low certainty due to serious imprecision and for using a subgroup that was not defined in advance. The confidence intervals were extremely wide for all important outcomes and included both appreciable harm and benefit.
The PREVER‐treatment 2016 trial, which used a combination therapy with potassium‐sparing diuretic (monotherapy: 84 participants; combination therapy: 116 participants), included outpatients. This trial was conducted in Brazil and had a follow‐up of 18 months. The number of events was very low and confidence intervals very wide, with zero events reported for cardiovascular mortality and withdrawals due to adverse events. It is very uncertain if there are differences in clinical outcomes between monotherapy and combination therapy in this trial.
Authors' conclusions
The numbers of included participants, and hence the number of events, were too small to draw any conclusion about the relative efficacy of monotherapy versus combination therapy, as initial treatment for primary hypertension. There is a need for large clinical trials that address the review question and report clinically relevant endpoints.
Plain language summary
Beginning treatment of hypertension with one medicine versus with a combination of two medicines
What is high blood pressure?
This is the second update of a review published for the first time in 2017. Hypertension (high blood pressure) is a long‐term condition that increases the risk of health problems such as heart attack, stroke, or kidney disease.
How is high blood pressure treated?
Several types of medicines are used to treat hypertension. Over time a person with hypertension will often need more than one type of medicine to help control their blood pressure. A doctor prescribing medicines to reduce blood pressure for the first time in a patient has two options: using only one medicine (monotherapy) or using two medicines (combination therapy). Combination therapy can be in the same tablet or in different tablets.
What did we want to find out?
We wanted to find out if there are differences between treating people with high blood pressure with one medicine or with two medicines. The potential advantage of using two medicines is that blood pressure may fall faster, but we do not know if this is better or worse for avoiding harmful effects in the patient.
What did we do?
We searched for studies that compared starting treatment of hypertension in adults with one medicine versus studies that started treatment with two medicines. Studies had to talk about results such as deaths or other events caused by diseases of the heart or the blood vessels, such as heart attack, stroke, or heart failure. Studies could also talk about other kinds of health‐related side effects. We only chose studies with 50 or more people in each group and that lasted at least 12 months.
What did we find?
In this update we did not find any new studies, with a total of four studies included in the review, with 419 treated with one medicine and 349 people treated with more than one medicine. However, there was not enough information to answer our review question. There is a need for more and larger studies that compare using one medicine versus using two medicines as the first treatment for high blood pressure.
Summary of findings
Summary of findings 1. Combination therapy compared to monotherapy for primary hypertension.
| Combination therapy compared to monotherapy for primary hypertension | |||||
| Patient or population: people with primary hypertension Setting: outpatients mostly in Europe Intervention: combination therapy (verapamil/trandolapril, perindopril/indapamide) Comparison: monotherapy (verapamil, trandolapril, enalapril, atenolol) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with monotherapy | Risk with combination therapy | ||||
|
Total mortality Follow‐up: 12 to 36 months |
3 per 1000 | 4 per 1000 (0 to 65) | RR 1.35 (0.08 to 21.72) | 568 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c |
|
Cardiovascular mortality Follow‐up: 12 to 36 months |
0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 568 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,d |
|
Cardiovascular events Follow‐up: 12 to 36 months |
9 per 1000 | 9 per 1000 (2 to 39) | RR 0.98 (0.22 to 4.41) | 568 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c |
|
Serious adverse events Follow‐up: 12 to 36 months |
176 per 1000 | 136 per 1000 (55 to 338) | RR 0.77 (0.31 to 1.92) | 568 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,e |
|
Withdrawals due to adverse effects Follow‐up: 12 to 36 months |
128 per 1000 | 109 per 1000 (68 to 173) | RR 0.85 (0.53 to 1.35) | 568 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,e |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for serious risk of bias because all data came from subgroups of participants not predefined in the original studies, and the outcomes of our review were not the primary outcome in any included trial. bWe downgraded by one level for serious indirectness because two trials included only people with type 2 diabetes, whereas the other trial excluded participants treated with drugs for diabetes, hypocholesterolaemia, or cardiovascular disease, so none of these studies was fully representative of the general hypertensive population. cWe downgraded by two levels for very serious imprecision because there were very few events and confidence intervals were extremely wide. dWe downgraded by two levels for very serious imprecision because there were no events for this outcome. eWe downgraded by two levels for very serious imprecision because confidence intervals were wide and included both appreciable harm and appreciable benefit.
Summary of findings 2. Combination with potassium‐sparing diuretics versus monotherapy for primary hypertension.
| Combination with potassium‐sparing diuretics versus monotherapy for primary hypertension | |||||
| Patient or population: people with primary hypertension Setting: outpatients in Brazil Intervention: combination with potassium‐sparing diuretics (chlorthalidone/amiloride) Comparison: monotherapy (losartan) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with monotherapy | Risk with combination with potassium‐sparing diuretics | ||||
|
Total mortality Follow‐up: 18 months |
12 per 1000 | 3 per 1000 (0 to 70) | RR 0.24 (0.01 to 5.87) | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b |
|
Cardiovascular mortality Follow‐up: 18 months |
0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c |
|
Cardiovascular events Follow‐up: 18 months |
12 per 1000 | 17 per 1000 (2 to 187) | RR 1.45 (0.13 to 15.71) | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b |
|
Serious adverse events Follow‐up: 18 months |
24 per 1000 | 17 per 1000 (2 to 120) | RR 0.72 (0.10 to 5.04) | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b |
|
Withdrawals due to adverse effects Follow‐up: 18 months |
0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 200 (1 RCT) | ⊕⊝⊝⊝ VERY LOWWa,c |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for serious risk of bias because all data came from a subgroup of participants not predefined in the original study, and outcomes of our review were not the primary outcome in the trial. bWe downgraded by two levels for very serious imprecision because there were very few events and confidence intervals were extremely wide. cWe downgraded by two levels for very serious imprecision because there were no events for this outcome.
Background
Description of the condition
Elevated blood pressure (hypertension), arbitrarily defined as systolic blood pressure of 140 mmHg or greater or diastolic blood pressure of 90 mmHg or greater, is a risk factor for stroke, myocardial infarction, renal failure, congestive heart failure, and peripheral artery disease. There is a graded relationship between blood pressure and the risk of cardiovascular disease (ESH/ESC 2018; NICE 2019). Approximately 90% of cases of elevated blood pressure are considered to be primary hypertension, as there is no secondary cause that can be determined (ESH/ESC 2018). The main goal of attempts to lower the blood pressure is to prevent cardiovascular morbidity and death, without adversely affecting quality of life. Blood pressure reduction per se is one of the main approaches to cardiovascular risk reduction (Gradman 2010; Law 2009).
Description of the intervention
Stepped therapy constitutes the usual initial approach in most people with hypertension, increasing the dose of the first drug or adding other drugs if blood pressure targets are not reached. First‐line low‐dose thiazides have the best evidence for reducing mortality and morbidity (Wright 2018). Guidelines that are often based on lower levels of evidence have suggested other classes for first‐line therapy in addition to thiazides, including: beta‐blockers, angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) (ESH/ESC 2018; Hypertension Canada 2020). They also suggest that determination of the need for drug therapy is based on a combined assessment of the blood pressure level and the risk for cardiovascular disease. Available data suggest that at least 75% of people with hypertension will require combination therapy to achieve blood pressure targets (Gradman 2010).
The aim of using a combination of two antihypertensive drugs as initial therapy is providing a faster reduction in blood pressure. The preferred drug combinations are ACEI or ARB with a thiazide‐type diuretic or CCB, and thiazide‐type diuretic with CCB (ESH/ESC 2018; Hypertension Canada 2020). In one large trial with high‐risk participants, ACEI/CCB combination resulted in fewer cardiovascular events than ACEI/hydrochlorothiazide (ACCOMPLISH 2008). Guidelines have suggested that chlorthalidone and indapamide have better evidence of benefit on clinical outcomes than bendroflumethiazide or hydrochlorothiazide (NICE 2019); however, this is not supported by evidence from randomised controlled trials (Wright 2018). Furthermore, most single‐pill combinations include hydrochlorothiazide. The combination of ACEI and ARB is not recommended (ESH/ESC 2018; Hypertension Canada 2020; JNC 8 2014; NICE 2019; Whelton 2018).
How the intervention might work
Treatment of hypertension reduces the risk of stroke, coronary artery disease, and congestive heart failure, as well as overall cardiovascular morbidity and mortality from cardiovascular causes. Stepped antihypertensive therapy starting with low‐dose thiazides reduces mortality and cardiovascular morbidity (Wright 2018), and in head‐to‐head trials, first‐line thiazides are better at reducing total cardiovascular events than first‐line beta‐blockers (Wiysonge 2017), first‐line drugs inhibiting the renin‐angiotensin system (Chen 2018), and first‐line CCBs (Zhu 2021).
The clinical practice guideline from the National Institute for Health and Care Excellence (NICE) recommends monotherapy as the initial approach, even for people with type 2 diabetes (NICE 2019). Some current guidelines suggest that two drugs be used for initial therapy if there is an elevation in blood pressure of 20 mmHg systolic or 10 mmHg diastolic above goal (JNC 8 2014; Whelton 2018). The guideline from the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) recommends initiating an antihypertensive treatment with a two‐drug combination of ACEI or ARB plus CCB or diuretic, with a subsequent increase in drug number, if needed. Monotherapy may be considered as the initial treatment for frail older patients and those at low risk and with grade 1 hypertension (particularly if systolic blood pressure is less than 150 mmHg) (ESH/ESC 2018). For people with past stroke or transient ischaemic attack, Hypertension Canada 2020 recommends initiating treatment with an ACEI/thiazide‐type diuretic combination.
Some advantages of initial combination treatment have been proposed. Two drugs can be given at low doses, thereby reducing the risk of adverse effects. Combination therapy provides more rapid control of blood pressure than monotherapy. Adherence may be improved, and subsequent blood pressure control, when the person perceives the treatment is effective and well tolerated (ESH/ESC 2018).
The disadvantages of initiating treatment with drug combinations are that one of the drugs may be ineffective or unnecessary, thus complicating the treatment (ESH/ESC 2013), and that a substantial decrease in blood pressure can be poorly tolerated in some people (e.g. older people).
Why it is important to do this review
It is unknown whether the benefits of combination therapy compared to monotherapy for initial treatment of hypertension exceed the harms.
One meta‐analysis showed that combining drugs from different classes is more effective in lowering blood pressure than increasing the dose of one drug, but it did not provide information about morbidity or adverse effects. The authors recommended considering combination therapy as routine initial therapy (Wald 2009). Although the value of routinely starting treatment with combination therapy, particularly with low doses, has been proposed, it has not been widely accepted. It is unknown if beginning with two drugs results in improved cardiovascular outcomes or mortality compared with starting with one drug (JNC 8 2014). The recommendation to use combinations when blood pressure is 20/10 mmHg above goal is not based on direct evidence from randomised controlled trials (JNC 8 2014; Whelton 2018).
Combinations of drugs acting on the renin‐angiotensin system have been proposed, but these have been shown to be harmful (ALTITUDE 2012; Makani 2013; ONTARGET 2008).
One observational study including 1127 people older than 80 years living in nursing homes found a significant increase in two‐year mortality (adjusted hazard ratio 1.78, 95% confidence interval (CI) 1.34 to 2.37) associated with combinations of antihypertensive drugs in people with systolic blood pressure less than 130 mmHg (PARTAGE 2015). Other observational studies have claimed benefits with combination strategy versus monotherapy: Rea 2018 (44,534 participants aged 40 to 80 years) showed a reduced one‐year risk of hospitalisation for cardiovascular events (hazard ratio 0.85, 95% CI 0.74 to 0.97) after matching by a high‐dimensional propensity score. Weir 2017 (48,131 participants, median age 57 years) showed greater odds of achieving blood pressure control at six months (odds ratio 1.21, 95% CI 1.09 to 1.35).
There is an increasing awareness about the problem of polypharmacy. The single most important predictor of risk of adverse drug events in older people is the number of prescribed drugs, so using the minimum number of drugs is a measure to improve patient safety (Scott 2015).
Objectives
To determine if there are differences in clinical outcomes between monotherapy and combination therapy as initial treatment for primary hypertension.
Methods
Criteria for considering studies for this review
Types of studies
Double‐blind randomised controlled trials of at least one‐year duration and containing 50 or more participants per group. The trials must have reported data for at least one of the primary outcomes. We excluded trials using non‐randomised allocation methods such as alternate allocation, week of presentation, or retrospective controls.
Types of participants
We included participants aged at least 18 years whose blood pressure was measured using a validated technique.
Trials were limited to those in which participants had a baseline resting systolic blood pressure of at least 140 mmHg or a diastolic blood pressure of at least 90 mmHg (130/80 mmHg or greater in people with diabetes). We included the study if 70% or more of the participants met the above definitions; individual participant data were available; or data of relevant participants were provided separately, allowing inclusion of this specific population.
We excluded people with confirmed secondary hypertension.
Trials were not limited by any other factor or baseline risk.
Types of interventions
Intervention: combination therapy (i.e. participants treated initially with two antihypertensive drugs).
Control: monotherapy (i.e. participants treated initially with one antihypertensive drug).
Treatment should have been clearly defined as a specific class of first‐line antihypertensive therapy in one of the following classes: thiazide‐type diuretics, loop diuretics, beta‐blockers, CCBs, ACEIs, ARBs, renin inhibitors, or α‐adrenergic blockers (ATC codes: C03, C07, C08, C09, C02CA, C02LE). We excluded drug classes that have not been confirmed to lower blood pressure as monotherapy (e.g. potassium‐sparing diuretics triamterene and amiloride) (Heran 2012). We planned that if a trial used a combination of diuretics with these agents, we would analyse them separately. Aldosterone antagonists (spironolactone and eplerenone) are normally used for resistant hypertension (ESH/ESC 2018; Whelton 2018). Trials using a combination of first‐line agents with aldosterone antagonists would also be included as combination therapy.
Initial therapy is defined as the first time participants were treated with antihypertensive drugs.
Both groups under study should have had the same blood pressure target, if this was defined.
Drugs and doses were acceptable when the doses were within the manufacturer‐recommended dose range for hypertension.
Types of outcome measures
Primary outcomes
Total mortality.
Total serious adverse events, defined according to the International Conference on Harmonisation Guidelines (ICH 1995), as any event that leads to death, was life‐threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability, or that was a congenital anomaly/birth defect.
Total cardiovascular events including total myocardial infarction, stroke, sudden death, hospitalisation or death from congestive heart failure, and other significant vascular events such as ruptured aneurysms (not including angina, transient ischaemic attacks, surgical or other procedures, or accelerated hypertension).
Cardiovascular mortality.
If a study used a different definition for serious adverse events, two review authors (JG and LCS) decided on inclusion of the data by consensus, consulting another review author where required. .
All primary outcomes were important outcomes to be included in the summary of findings table.
Secondary outcomes
Withdrawals due to drug‐related adverse effects (important outcome).
Reaching blood pressure control, as defined in each trial.
Systolic and diastolic blood pressure change from baseline.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches of the following databases for primary studies, without language or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 12 March 2021);
the Cochrane Central Register of Controlled Trials (CENTRAL, 2019, Issue 3) via CRS‐Web (searched 11 March 2021);
MEDLINE Ovid (from 1946), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 11 March 2021);
Embase (from 1974) (searched 11 March 2021);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/) (searched 12 March 2021);
WHO International Clinical Trials Registry Platform (ICTRP) via CENTRAL in CRS‐Web (searched 12 March 2021)
The Specialised Register also includes searches for controlled trials in the Allied and Complementary Medicine Database (AMED), CAB Abstracts & Global Health, Cumulative Index to Nursing and Allied Health Literature (CINAHL), ProQuest Dissertations & Theses, and Web of Science.
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, these were combined with subject strategy adaptations of the sensitivity‐ and precision‐maximising search strategy designed by Cochrane for identifying randomised controlled trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). The search strategies for major databases are shown in Appendix 1.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches for controlled trials in AMED, CAB Abstracts & Global Health, CINAHL, ProQuest Dissertations & Theses, and Web of Science.
We contacted relevant pharmaceutical companies and searched their clinical studies repositories (Appendix 2).
We searched the websites of the US Food and Drug Administration (FDA) (www.fda.gov) and the European Medicines Agency (EMA) (www.ema.europa.eu) for published and unpublished clinical trial data relevant to this review. We only considered FDA and EMA reports of authorised combination drugs. We searched the Scientific Discussion of the European Public Assessment Reports and the FDA's Medical Reviews.
We searched the TRIP Database (www.tripdatabase.com) for systematic reviews, guidelines, and health technology assessment reports.
We searched for additional trials by checking the reference lists of included trials and reviews, guidelines, and health technology assessment reports (Appendix 3).
Dealing with duplicate publications
When we identified more than one publication of an original trial, we assessed the articles together to maximise data collection.
References from published studies
We examined the references of the included and excluded studies for further potentially eligible randomised controlled trials.
Language
We applied no language restrictions.
Correspondence
We contacted trial investigators to request data for the subgroup of participants without previous antihypertensive treatment, for missing data, or to clarify study details.
Data collection and analysis
Two review authors independently reviewed the search results. One review author (JG) checked all results and the remaining review authors (LCS, AA, IG, MJA, JE) participated as a second review author by dividing the whole search results among themselves. We used EROS and Covidence software for screening and classifying references.
Selection of studies
We performed an initial screening based on title and abstract, excluding records for any of the following reasons:
not a double‐blind randomised controlled trial;
participants were not naïve to antihypertensive treatment;
there were fewer than 50 participants per group;
follow‐up was less than 12 months;
the trial did not compare monotherapy with a combination therapy of the included classes;
blood pressure targets differed between groups; or
antihypertensive doses were not in the recommended range.
We obtained the full text of the remaining articles and assessed whether they met the inclusion criteria. If we determined that a study could have included a subgroup of participants that met our inclusion criteria (more than 50 people with hypertension without previous antihypertensive drugs per group), we provisionally included it and contacted the study authors for data for the subgroup.
Two review authors independently reviewed the selected articles. One review author (LCS) checked all results and the remaining review authors (JG, AA, IG, MJA, JE) participated as a second review author by dividing the whole search results among themselves. Any disagreements were resolved by discussion or by involving all review authors if necessary.
Data extraction and management
Two review authors (JG and LCS) independently extracted data from the included trials, using a standardised data extraction form which included the following:
study design;
randomisation;
allocation concealment;
blinding;
drugs;
doses;
duration of treatment;
baseline characteristics;
losses to follow‐up;
outcomes;
analysis and reporting.
Any disagreements between authors were resolved by discussion or by involving all review authors if necessary.
We used Access 2010 and Excel 2010 for dealing with individual participant data.
We used Review Manager 5 software for data synthesis and analyses (Review Manager 2020). We based quantitative analyses of outcomes on the intention‐to‐treat principle.
We considered all publications of the trials, including protocols and FDA‐ and EMA‐authorised drug reports.
Assessment of risk of bias in included studies
Two review authors independently assessed the risks of bias in each trial using the Cochrane risk of bias tool (Higgins 2021). One review author (LCS) checked all results and the remaining review authors (JG, AA, IG, MJA, JE) participated as a second review author by dividing the whole search results among themselves. Any disagreements were resolved by discussion or by consulting a third review author if necessary.
We reported the overall risk of bias for each of the included studies according to the following:
low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met;
unclear risk of bias (plausible bias that raised some doubt about the results) if one or more criteria were assessed as unclear;
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
We planned to perform sensitivity analyses excluding trials with high or high and unclear risk of bias.
Measures of treatment effect
We based quantitative analyses of outcomes on intention‐to‐treat results.
We statistically summarised data on dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). We planned to calculate the risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB).
We summarised continuous outcomes as mean differences (MDs) with a 95% CI.
We combined data for change in blood pressure using mean differences.
Unit of analysis issues
The unit of analysis was individual participants.
Dealing with missing data
We contacted study investigators in the case of missing data. We based the quantitative analyses of outcomes on intention‐to‐treat results.
Assessment of heterogeneity
We planned to examine heterogeneity using the standard Chi2 test and the I2 statistic.
We assessed values of the I2 statistic as follows (Higgins 2021):
0% to 40%: heterogeneity might not be important;
30% to 60%: moderate heterogeneity;
50% to 90%: substantial heterogeneity;
75% to 100% considerable heterogeneity.
We planned that if data exhibited more than moderate heterogeneity (I2 greater than 60%), we would investigate possible causes. If the causes of the heterogeneity could not be addressed, we would not perform meta‐analysis.
Assessment of reporting biases
We planned to assess reporting bias following the recommendations on testing for funnel plot asymmetry, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Data synthesis
Two review authors analysed the data in Review Manager 5 (Review Manager 2020), and reported them in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
We planned to use a fixed‐effect model to pool the data in a meta‐analysis. In the presence of statistical heterogeneity (greater than 30%, or P < 0.05 as estimated by the I2 statistic), we used a random‐effects model.
If meta‐analysis was not appropriate, we would describe the results narratively.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses:
people aged less than 75 years versus people aged 75 years or over;
men versus women;
people with diabetes versus people without diabetes.
Sensitivity analysis
We planned to perform sensitivity analyses to assess the robustness of the results, as follows.
-
According to baseline blood pressure levels:
less than 160 mmHg;
160 mmHg and over to less than 180 mmHg;
180 mmHg and over.
Pharmaceutical‐sponsored versus independent trials. We considered a trial as pharmaceutical sponsored if this was noted in the publication; if any of the authors worked for a pharmaceutical company; or if the trial was sent to the FDA or EMA for drug authorisation.
Excluding trials with high or high and unclear risk of bias.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions, along with GRADE Pro GDT software (GRADEpro GDT; Higgins 2021). The outcomes included:
• Total mortality; • Cardiovascular mortality; • Cardiovascular events; • Serious adverse events; • Withdrawals due to adverse effects;
We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data for the prespecified outcomes. We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid the reader’s understanding of the review where necessary.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification tables.
Results of the search
We identified 29,834 records from our searches of the databases and 48 records from additional sources. There remained 12,637 records after removal of duplicates. We screened the titles and abstracts of these records, excluding 12,402 records. We obtained the full‐text articles of 235 records and assessed these for eligibility. We excluded 189 full‐text articles. We provisionally accepted 14 studies (reported in 46 articles) for inclusion whilst we contacted authors for subgroup data. We subsequently included four studies, excluded four studies, and listed six studies as awaiting classification. See Figure 1 for the flow chart of the bibliographic search.
1.
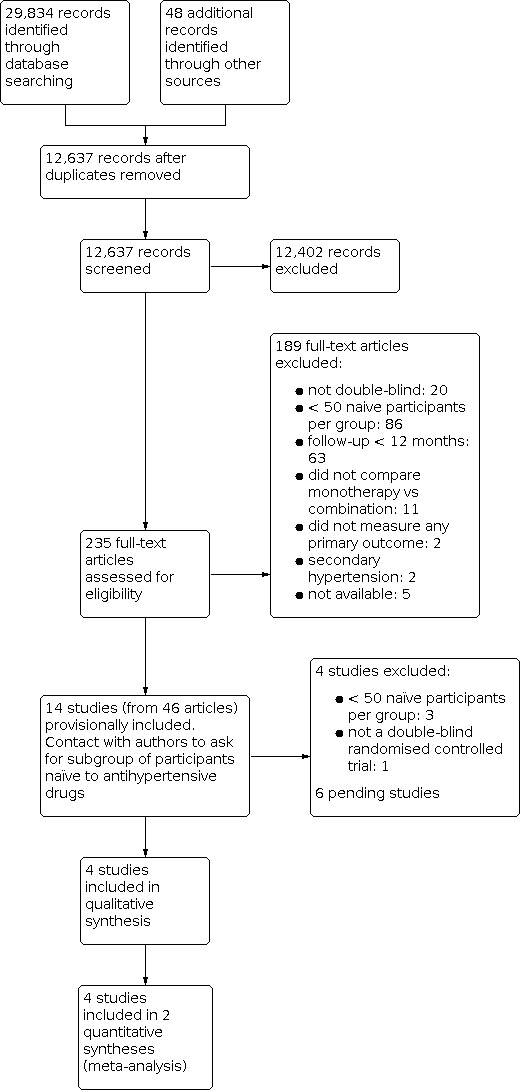
Study flow diagram.
We sought help in checking the inclusion criteria of two full‐text articles written in Chinese and Russian.
We checked 100 clinical studies included in the FDA's Medical Reviews of 24 fixed‐combination drugs, and 70 clinical studies included in the EMA's scientific discussion of the Public Assessment Reports of seven fixed‐combination drugs. None of these studies met our inclusion criteria, primarily due to follow‐up of less than one year. We did not check whether studies included in FDA or EMA reviews were also in the bibliographic search.
Included studies
https://revman.cochrane.org/#/422712022703251559/sofTables#396502466839899200. We found no additional trials for this update. The three originally‐included studies involved 1867 participants with active treatment (monotherapy: 1077 participants; combination: 790 participants). None of the studies was limited to people who initiated antihypertensive treatment, so we asked investigators for this subgroup, which comprised 568 participants, 30% of the total (monotherapy: 335 participants; combination: 233 participants). PREMIER 2003 and BENEDICT‐A 2004 included only people with type 2 diabetes. PREMIER 2003 included only people with albuminuria, whereas BENEDICT‐A 2004 excluded people with albuminuria. REASON 2001 excluded people treated with antidiabetes, hypocholesterolaemia, or cardiovascular drugs. The characteristics of participants included in the review are shown in Table 3. Follow‐up was 12 months in PREMIER 2003 and REASON 2001, and 36 months in BENEDICT‐A 2004. The following therapeutic groups were compared: ACEI/thiazide‐type diuretic versus ACEI (PREMIER 2003); ACEI/thiazide‐type diuretic versus beta‐blocker (REASON 2001); and ACEI/CCB (non‐dihydropyridine) versus ACEI or CCB (non‐dihydropyridine) (BENEDICT‐A 2004). The three trials were industry‐funded.
1. Baseline characteristics of included participants (without previous antihypertensive treatment).
| Characteristic | Treatment | Mean (standard deviation) | |||
| BENEDICT‐A 2004 | PREMIER 2003 | REASON 2001 | PREVER‐treatment 2016 | ||
| Number of participants | Combination | 115 | 55 | 63 | 116 |
| Monotherapy | 215 | 54 | 66 | 84 | |
| Total participants included in the trial (%) | Combination | 38.08% | 22.78% | 28.09% | 34.83% |
| Monotherapy | 35.54% | 22.54% | 25.82% | 26.09% | |
| Age (years) | Combination | 60.98 (7.62) | 57.27 (8.53) | 52.49 (12.68) | 51.8 (8.2) |
| Monotherapy | 60.62 (8.36) | 59.93 (8.75) | 50.38 (10.57) | 54.0 (9.0) | |
| Sex (% men) | Combination | 67.83% | 74.55% | 71.43% | 56.90% |
| Monotherapy | 69.30% | 77.78% | 62.12% | 61.90% | |
| Ethnicity (% white people) | Combination | 100.00% | 96.36% | 98.41% | 62.1% |
| Monotherapy | 100.00% | 88.89% | 93.94% | 64.3% | |
| Body mass index (kg/m2) | Combination | 28.68 (5.19) | 28.23 (3.18) | 26.85 (3.11) | 29.1 (5.0) |
| Monotherapy | 28.34 (4.42) | 29.22 (3.51) | 26.99 (2.38) | 28.8 (4.7) | |
| Systolic blood pressure (mmHg) | Combination | 151.61 (9.70) | 154.56 (9.86) | 162.56 (11.24) | 140.4 (8.8) |
| Monotherapy | 152.11 (11.57) | 154.04 (11.67) | 158.74 (12.84) | 142.0 (8.4) | |
| Diastolic blood pressure (mmHg) | Combination | 88.72 (7.17) | 90.98 (8.43) | 97.65 (6.89) | 91.7 (7.4) |
| Monotherapy | 89.54 (6.32) | 91.00 (8.26) | 98.94 (5.07) | 90.3 (7.0) | |
PREVER‐treatment 2016 included 655 participants with no current use of antihypertensives and no previous cardiovascular disease. Cardiovascular events was a prespecified secondary outcome. Follow‐up was 18 months. We asked for data for participants who were naïve to antihypertensives, of which there were 200 (monotherapy: 84; combination: 116) (Table 3), 14 (monotherapy: 7; combination: 7) of whom had diabetes. The PREVER‐treatment 2016 study compared a combination pill of thiazide‐type diuretic/potassium‐sparing diuretic versus ARB monotherapy. As potassium‐sparing diuretics are not indicated in monotherapy, but included in the combination arm of this trial, we have presented the results for this study separately. PREVER‐treatment 2016 was not industry‐funded.
Excluded studies
As our objective was the first‐line therapy of hypertension, we discarded numerous studies that compared monotherapy with combination therapy because of failure of monotherapy.
One large study included only people who were not taking antihypertensive drugs. MRC‐O 1992 was conducted in general practices in the UK. Participants aged 65 to 74 years, with systolic blood pressure of 160 mmHg to 209 mmHg and diastolic blood pressure less than 115 mmHg, were randomised to atenolol 50 mg daily (1102 participants) or hydrochlorothiazide 25 mg to 50 mg daily plus amiloride 2.5 mg to 5 mg daily (1081 participants). The regimens were adjusted to achieve target systolic blood pressures of 150 mmHg or less or 160 mmHg or less, depending on baseline blood pressure. Mean follow‐up was 5.8 years. The participants in the combination‐therapy group had fewer cardiovascular deaths and fewer cardiovascular events. There were no statistically significant differences in total mortality (Wiysonge 2017). We excluded this trial because doctors and nurses were not blinded to treatment.
PICXEL 2005 included 556 participants with hypertension and left ventricular hypertrophy who were randomised to receive perindopril 2 mg plus indapamide 0.625 mg or enalapril 10 mg. Doses were increased depending on response. Follow‐up was 12 months. We sought data from participants without prior antihypertensive treatment from the study authors. However, we excluded this trial as there were fewer than 50 participants per group (perindopril/indapamide: 40 participants, enalapril: 46 participants).
DEMAND 2011 included 380 participants aged 40 years or over with hypertension and a known history of type 2 diabetes mellitus for less than 25 years, with urinary albumin excretion of less than 200 μg/minute and serum creatinine of 1.5 mg/dL or less. Participants were randomised to manidipine 10 mg daily plus delapril 30 mg daily, delapril 30 mg daily, or placebo. Target blood pressure was 120/80 mmHg. Additional antihypertensive drugs were allowed to achieve target blood pressure. The mean follow‐up was 47 months. The study authors provided individual participant data, but there were fewer than 50 participants without prior antihypertensive treatment per group (delapril/manidipine: 38 participants, delapril: 33 participants).
BENEDICT‐B 2011 included 281 participants aged 40 years or over with hypertension (defined as an untreated systolic blood pressure of 130 mmHg or greater or diastolic blood pressure of 85 mmHg or greater), history of type 2 diabetes mellitus not exceeding 25 years, urinary albumin excretion rate 20 μg/minute or greater and less than 200 μg/minute, and serum creatinine concentration of 1.5 mg/dL or less. Two categories of participants entered the study: people who had developed microalbuminuria during the BENEDICT‐A 2004 study, and people included after a new screening. Participants were randomised to trandolapril 2 mg daily or trandolapril 2 mg daily plus verapamil 180 mg daily. The target blood pressure was 120/80 mmHg. Additional antihypertensive drugs were allowed to achieve the target blood pressure. Median follow‐up was 4.5 years. The authors provided individual participant data, but there were fewer than 50 participants without prior antihypertensive treatment per group (trandolapril: 39 participants, trandolapril/verapamil: 40 participants).
ONTARGET 2008 included 25,620 participants with coronary, peripheral, or cerebrovascular disease or diabetes with end‐organ damage. Hypertension was not required for inclusion. Participants were randomised to ramipril 10 mg, telmisartan 80 mg, or ramipril 10 mg plus telmisartan 80 mg. We excluded this trial because there was a three‐week run‐in period in which participants received ramipril plus telmisartan, so participants were not naïve to antihypertensive treatment at randomisation.
Zhang 2010 included 124 participants, of which 112 had no history of using any antihypertensive medication, who were randomised to fosinopril/indapamide or fosinopril alone. Follow‐up was 14 months. We excluded this trial because it was not stated to be double‐blind and it did not evaluate any of the primary outcomes.
ACCELERATE 2011 enrolled 1254 participants, of whom 521 were treatment‐naïve, who were randomised to aliskiren 150 mg (a direct renin inhibitor), amlodipine 5 mg, or aliskiren 150 mg plus amlodipine 5 mg. We excluded this trial because follow‐up was only 32 weeks.
PATHWAY‐1 2017 included 605 participants, of whom 269 had never been previously treated for hypertension, who were randomised to monotherapy with losartan or hydrochlorothiazide or a combination of losartan plus hydrochlorothiazide. We excluded this trial because at week 17 all participants receive a forced open‐label combination, so the double‐blind comparison of monotherapy versus combination only lasted 16 weeks.
PREVER‐prevention 2016 randomised 730 participants (372 allocated to chlortalidone plus amiloride versus 358 allocated to placebo). We excluded this study due to its exclusive focus on pre‐hypertension, a condition not within the scope of this review.
Risk of bias in included studies
The assessment of risk of bias is based on both published and unpublished data. Study authors provided clarification of methods for PREMIER 2003 and REASON 2001 and the protocol of BENEDICT‐A 2004. The risk of bias summary of included studies is shown in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All four included studies used a computer‐generated randomisation list. The allocation was performed centrally, and study centres received blinded treatments and randomisation numbers. We judged the risk of allocation bias to be low for all included studies.
Blinding
All four included studies were double‐blind, and stated that capsules of identical appearance were used. REASON 2001, BENEDICT‐A 2004, and PREMIER 2003 reported that evaluators were blinded to treatment. PREVER‐treatment 2016 reported that participants, members of the steering committee, healthcare staff, data collectors, and outcome assessors were blinded to the intervention arm. We judged the risk of performance and detection bias to be low for all included studies.
Incomplete outcome data
There were 21 withdrawals in REASON 2001, the reasons for which were not provided. In PREMIER 2003, there were more withdrawals due to lack of efficacy in the monotherapy group (six with monotherapy versus zero with combination therapy). It is unclear if such circumstances could have led to differences in endpoints. BENEDICT‐A 2004 and PREVER‐treatment 2016 did not show a relevant imbalance in reported withdrawals.
Selective reporting
We sent our protocol to investigators asking for outcomes of interest for this review in the subgroup of participants naïve to antihypertensive drugs. Study authors provided aggregate data for REASON 2001, PREMIER 2003, and PREVER‐treatment 2016, and individual participant data for BENEDICT‐A 2004, so we judged selective reporting bias to be low for all studies.
Other potential sources of bias
The inclusion criteria of BENEDICT‐A 2004 were changed during the trial (from untreated blood pressure 140/90 mmHg or greater to 130/85 mmHg or greater). Blood pressure targets were also changed during the trial from 130/85 mmHg to 120/80 mmHg (protocol amendment 3; 27 May 1999).
All data came from subgroups of participants not predefined in the original studies. The outcomes of our review were not the primary outcome in any included trial.
Effects of interventions
Combination therapy compared to monotherapy for primary hypertension
According to the data summarised in the main summary of findings table (Table 1) we did not find any differences in any of the primary outcomes. The results for each individual outcome are presented as follows:
Total mortality: risk ratio (RR) 1.35, 95% confidence interval (CI) 0.08 to 21.72; 3 studies, 568 participants (Analysis 1.1).
1.1. Analysis.

Comparison 1: Combination therapy versus monotherapy, Outcome 1: Total mortality
Total serious adverse events: RR 0.77, 95% CI 0.31 to 1.92; 3 studies, 568 participants (Analysis 1.2).
1.2. Analysis.

Comparison 1: Combination therapy versus monotherapy, Outcome 2: Serious adverse events
Total cardiovascular events: RR 0.98, 95% CI 0.22 to 4.41; 3 studies, 568 participants (Analysis 1.3).
1.3. Analysis.

Comparison 1: Combination therapy versus monotherapy, Outcome 3: Cardiovascular events
Cardiovascular mortality: zero events in the three included studies (Analysis 1.4).
1.4. Analysis.

Comparison 1: Combination therapy versus monotherapy, Outcome 4: Cardiovascular mortality
Withdrawals due to adverse effects: RR 0.85, 95% CI 0.53 to 1.35; 3 studies, 568 participants (Analysis 1.5).
1.5. Analysis.

Comparison 1: Combination therapy versus monotherapy, Outcome 5: Withdrawals due to adverse effects
The large heterogeneity precluded aggregating results of 'reaching blood pressure control' (Analysis 1.6). Separating in subgroups by blood pressure target did not address this heterogeneity. Some explanation can be provided by differences in how trials were conducted. In REASON 2001, the dose was doubled after three months if blood pressure remained above 160/90 mmHg, whereas in PREMIER 2003 the dose was doubled after three months if blood pressure remained above 140/90 mmHg. Despite this, target blood pressure was defined as less than 140/90 mmHg in data provided by investigators in both trials. Another factor may be that REASON 2001 used atenolol as monotherapy, but it was not included in the combination therapy.
1.6. Analysis.

Comparison 1: Combination therapy versus monotherapy, Outcome 6: Reaching blood pressure control
At the end of one year, there were no statistically significant differences in change of systolic blood pressure (mean difference −2.06, 95% CI −5.39 to 1.27; 3 studies, 568 participants Analysis 1.7) or diastolic blood pressure (mean difference −0.12, 95% CI −1.21 to 0.96; 2 studies, 443 participants Analysis 1.8) between groups started with monotherapy or with combination therapy. However, the CIs included differences larger than 5 mmHg for systolic blood pressure.
1.7. Analysis.

Comparison 1: Combination therapy versus monotherapy, Outcome 7: Systolic blood pressure change from baseline at end of 1 year
1.8. Analysis.

Comparison 1: Combination therapy versus monotherapy, Outcome 8: Diastolic blood pressure change from baseline at end of 1 year
Only BENEDICT‐A 2004 provided separate results data for men and women. The scarcity of events for mortality, cardiovascular mortality, cardiovascular events, and 'reaching blood pressure control' precluded subgroup analysis for these outcomes. There was no indication of a different effect in serious adverse events (Analysis 2.1), withdrawals due to adverse effects (Analysis 2.2), or changes in blood pressure at one year (Analysis 2.3; Analysis 2.4). However, there were too few women to draw any conclusions. BENEDICT‐A 2004 also provided individual data for the age of participants, but as this study only included 17 people aged 75 years or older, data were insufficient to provide results for this subgroup.
2.1. Analysis.

Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 1: Serious adverse events
2.2. Analysis.

Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 2: Withdrawals due to adverse effects
2.3. Analysis.

Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 3: Systolic blood pressure change from baseline at end of 1 year
2.4. Analysis.

Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 4: Diastolic blood pressure change from baseline at end of 1 year
The number of participants included in our review, and hence the number of events, was clearly insufficient to reach any conclusion about the different effect of initiating treatment with combination therapy versus monotherapy on important outcomes. Similarly, we considered the scarce available information to be insufficient to support the aforementioned sensitivity analyses.
Combination with potassium‐sparing diuretics versus monotherapy for primary hypertension
A single study (PREVER‐treatment 2016) provided data for this comparison. According to the data summarised in Table 2, we did not find any differences in any of the primary outcomes. The results for each individual outcome are presented as follows:
Total mortality: RR 0.24, 95% CI 0.01 to 5.87; 1 study, 200 participants (Analysis 3.1).
3.1. Analysis.

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 1: Total mortality
Total serious adverse events: RR 0.72, 95% CI 0.10 to 5.04; 1 study, 200 participants (Analysis 3.2).
3.2. Analysis.

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 2: Serious adverse events
Total cardiovascular events: RR 1.45, 95% CI 0.13 to 15.71; 1 study, 200 participants (Analysis 3.3).
3.3. Analysis.

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 3: Cardiovascular events
Cardiovascular mortality: zero events (Analysis 3.4).
3.4. Analysis.

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 4: Cardiovascular mortality
Withdrawals due to adverse effects: zero events (Analysis 3.5).
3.5. Analysis.

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 5: Withdrawals due to adverse effects
Reaching blood pressure control: there were no statistically significant differences for this outcome. RR 1.15, 95% CI 0.93 to 1.42; 1 study, 200 participants (Analysis 3.6).
3.6. Analysis.

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 6: Reaching blood pressure control
In the trial using a combination of thiazide‐type diuretic/potassium‐sparing diuretic, little information was available and no differences were identified between the interventions for total mortality, serious adverse events, cardiovascular events or reaching blood pressure control. The absence of events for cardiovascular mortality and withdrawals due to adverse effects precluded any conclusion about the effect of the interventions. No sensitivity analysis was performed, due to a lack of information.
Discussion
Summary of main results
For the comparison of combination therapy versus monotherapy, the number of participants (568) and hence the number of events, was very small. This led to very wide confidence intervals for the risk ratio of total mortality (RR 1.35, 95% CI 0.08 to 21.72), cardiovascular mortality (zero events reported), cardiovascular events (RR 0.98, 95% CI 0.22 to 4.41), serious adverse events (RR 0.77, 95% CI 0.31 to 1.92), and withdrawals due to adverse events (RR 0.85, 95% CI 0.53 to 1.35).
For the comparison of combination with potassium‐sparing diuretics versus monotherapy (200 participants), there were also few events, as well as substantial imprecision in results: total mortality (RR 0.24, 95% CI 0.01 to 5.87), cardiovascular mortality (zero events reported), cardiovascular events (RR 1.45, 95% CI 0.13 to 15.71), serious adverse events (RR 0.72, 95% CI 0.10 to 5.04), and withdrawals due to adverse events (zero events reported).
Confidence intervals included both substantial benefit and harm of combination therapy or combination with potassium‐sparing diuretics compared to monotherapy.
Overall completeness and applicability of evidence
Despite the huge number of clinical trials with antihypertensive drug combinations, our search was nearly fruitless. The reasons for excluding studies merit consideration.
No naïve participants: clinical trials are not addressing questions that doctors face in everyday practice. The inclusion of participants with and without previous antihypertensive treatment facilitates recruitment but impairs the interpretation and applicability of results. Clinical practice guidelines address the question, but recommendations are based on indirect evidence at best.
Follow‐up less than 12 months: there are numerous trials of short duration (eight to 12 weeks) that mostly respond to regulatory requirements for pharmaceutical companies. Follow‐ups of this length are not sufficient to provide results of hard clinical outcomes in a chronic condition like hypertension.
The dangers of relying only on theoretical or pharmacological considerations are well illustrated with the case of combinations of drugs targeting the renin‐angiotensin system. Some authors have recommended these drugs because they act at different levels of the physiological pathway and could have synergic actions. However, when those combinations were compared with monotherapy in large clinical trials with hard endpoints, the results were more adverse effects (including hypotension, hyperkalaemia, and renal failure), with no benefits in people without heart failure, despite greater reductions in blood pressure (ALTITUDE 2012; Makani 2013; ONTARGET 2008). Those results led regulatory agencies to amend product information to say that the combined use of ACEIs, ARBs or aliskiren is not recommended (EMA/294911/2014; FDA 2014).
Quality of the evidence
Although the included trials were at low risk of bias, the overall certainty of the evidence was very low. The reasons for downgrading the certainty of evidence were as follows.
Risk of bias due to using subgroups that were not defined in advance. On the other hand, our review outcomes were not defined as a primary outcome for any included trial.
Indirectness due to two trials including only people with type 2 diabetes, whereas another trial excluded participants treated with drugs for diabetes, hypocholesterolaemia or cardiovascular disease, so these trials were not fully representative of the hypertensive population.
Imprecision, because very few events were reported and confidence intervals were wide. Trials were underpowered to assess mortality or cardiovascular events. The need to select the subgroup of participants naïve to antihypertensives further reduced the sample size.
Potential biases in the review process
As stated above, one potential bias introduced into the review process was that we could not use the whole population of the trials, but only a subgroup.
We excluded MRC‐O 1992, which was not double‐blind. It could be argued that the evidence from this study may be as uncertain as that from the small subgroups of participants of the included trials. In the discussions that occurred during the design of our protocol, we decided to limit the inclusion criteria to double‐blind trials because we believed there was a high risk of different care or co‐interventions if doctors judged that participants were receiving treatments of different intensity.
Agreements and disagreements with other studies or reviews
In one large clinical trial that focused on people who were not taking antihypertensive drugs (MRC‐O 1992), there were better results in terms of cardiovascular morbidity and mortality with combination therapy than with monotherapy. There were no statistically significant differences between groups for all‐cause mortality. The drugs compared in the trial were hydrochlorothiazide/amiloride versus a beta‐blocker (atenolol) (see Excluded studies). It is unclear if the differences could have arisen from the different classes of drugs used. However, one systematic review did not find differences between diuretics and beta‐blockers for those outcomes (Wiysonge 2017), so the use of a combination of two diuretics with different mechanisms of action could have influenced the results. We did not include this trial because it was not double‐blind.
One systematic review included 42 clinical trials of a factorial design with durations of between four and 12 weeks (Wald 2009). One review included 354 trials (50 studying combination therapy) (Law 2003a; Law 2003b). The median duration was four weeks (range two to 15 weeks). The authors of these studies did not find any trials of sufficient duration to meet our inclusion criteria. They concluded that combination therapy is the preferred initial strategy in the treatment of hypertension (Law 2003a), but this statement was not based on results of hard clinical endpoints with combination therapy but on indirect evidence. They based their conclusions on the larger reduction of blood pressure obtained with combination therapy. However, we found no differences in blood pressure reductions attained after one year.
The JNC 8 2014 guideline supports both strategies: start with monotherapy, and begin with two drugs either as separate tablets or as a single tablet combination. The guideline's evidence review found no randomised controlled trials that compared monotherapy versus combination therapy and that assessed important health outcomes. The guideline acknowledges that it is unknown if one of the strategies results in improved cardiovascular, cerebrovascular, or kidney outcomes or mortality compared with the alternative strategy.
The guidelines of the American College of Cardiology, the American Heart Association, and several other organisations recommend the initiation of antihypertensive therapy with two first‐line agents of different classes in adults with stage 2 hypertension and an average blood pressure more than 20/10 mmHg above their target (Whelton 2018). These guidelines support the initiation with a single antihypertensive drug in adults with stage 1 hypertension, with dosage titration and the sequential addition of other agents to achieve the target. These statements are based on expert opinion, since the systematic review performed in support of this guideline (Reboussin 2018) compared the initiation of antihypertensive treatment with monotherapy and sequential (stepped‐care) titration of additional agents versus initiation of treatment with combination therapy, but it did not identify any randomised controlled trial meeting the systematic review questions.
The ESH/ESC 2018 guideline recognises that no randomised controlled trial has compared major cardiovascular outcomes between initial combination therapy and monotherapy. In spite of this, it makes a strong recommendation to initiate an antihypertensive treatment with a two‐drug combination, with the exceptions of frail older people and those at low risk and with stage 1 hypertension.
The NICE 2019 guideline recommends starting with monotherapy. Its evidence review addresses the question "Is monotherapy or combination antihypertensive therapy more clinically and cost effective for step 1 treatment for hypertension?". The population comprises adults with primary hypertension who are not on current pharmacological treatment for hypertension (minimum wash‐out four weeks), unlike our review, which includes only naïve participants. The NICE guideline includes PREMIER 2003, REASON 2001, and PICXEL 2005 trials. It found no statistically significant differences, with very low‐certainty evidence for the outcomes of serious cardiovascular events and discontinuation due to adverse events.
Recent retrospective cohort studies have performed comparisons between initiating antihypertensive treatment with monotherapy or with combination therapy. A study promoted by a pharmaceutical company that used the The Health Improvement Network UK general practice database (THIN) found that 96% of patients were initiated on monotherapy. The odds of achieving blood pressure control were higher in those initiating treatment with combination therapy. Surprisingly, patients with diabetes and those with grade 2 to 3 hypertension or with high normal/grade 1 hypertension plus at least one cardiovascular condition pretreatment were less likely than patients with no risk factors to receive combination therapy (Weir 2017). Another study, using the healthcare use database of the Lombardy Region (Italy), compared starting treatment with one antihypertensive drug versus a two‐drug fixed‐dose combination (patients who started with a free drug combination were excluded). This study was funded by a pharmaceutical company. Eighty‐three per cent of patients started treatment with one drug. Patients with combination therapy less frequently had a history of hospitalisation for cardiovascular diseases. Starting with combination therapy had a lower one‐year risk of hospitalisation for cardiovascular events (hazard ratio 0.85, 95% CI 0.74 to 0.97) after matching by a high‐dimensional propensity score (Rea 2018).
Our review confirms the lack of evidence from randomised controlled trials addressing this question. In addition, the possibility of increasing cost without evidence of benefit must be considered, especially in low‐income environments.
Authors' conclusions
Implications for practice.
In this second update of our review we found no new trials and therefore no changes to our conclusions. Doctors should be aware that recommendations about initiating antihypertensive therapy with drug combinations are not based on evidence from randomised controlled trials. This review demonstrates that the existing evidence is insufficient to distinguish between the two approaches: initiating therapy with a two‐drug combination or initiating therapy with one drug.
Implications for research.
There is a clear need for trials comparing monotherapy versus combination therapy as the initial treatment for hypertension. These trials need to be of sufficient duration and size to assess mortality and morbidity. Trials including people with and without previous antihypertensive treatment should provide separate results for these groups. People of particular interest are those with complicated hypertension and those whose blood pressure is more than 20/10 mmHg above their goal blood pressure. Older people and different ethnic groups should be well represented, due to possible differences in response to drugs. The most obvious approach that should be studied is a combination thiazide‐type diuretic plus angiotensin‐converting enzyme inhibitor versus thiazide‐type diuretic alone.
What's new
| Date | Event | Description |
|---|---|---|
| 24 June 2021 | Amended | We updated the search in March 2021 and included no additional studies. We cite the current version of Hypertension Canada 2020 guideline in the Background and Discussion sections. We cite the current Cochrane Handbook (Higgins 2021). We added a reference for the PREVER‐treatment 2016 trial. Results and conclusions remain unchanged. |
History
Protocol first published: Issue 1, 2013 Review first published: Issue 1, 2017
| Date | Event | Description |
|---|---|---|
| 5 February 2020 | New citation required but conclusions have not changed | review updated |
| 4 February 2020 | New search has been performed | A pending study was included without changing the conclusions. We used the current versions of Cochrane Reviews and clinical practice guidelines and incorporated a new guideline and two cohort studies in the Background and Discussion. We use the NICE 2019 guideline in the Background and Discussion. It replaces the reference to the 2011 NICE guideline on hypertension and the 2015 NICE guideline on type 2 diabetes. |
| 8 November 2013 | Amended | Adding the following text to Types of interventions to clarify the clinical sense of the potential results: "Combination therapy includes the combinations of diuretics with the potassium‐sparing agents triamterene or amiloride, but in any case we will analyse their data also separately because they are not used as antihypertensives in monotherapy". |
Acknowledgements
We are grateful to Dr James M Wright and the Cochrane Hypertension Group for their encouragement, support, and assistance.
Annalisa Perna, Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy, provided protocol and individual participant data for three studies (BENEDICT‐A 2004; BENEDICT‐B 2011; DEMAND 2011).
Institut de Recherches Internationales Servier, Courbevoie, France, and Prof Roland Asmar, Foundation ‐ Medical Research Institutes, Geneva, Switzerland, provided aggregate results of participants without previous antihypertensive treatment for three studies (REASON 2001; PREMIER 2003; PICXEL 2005).
Flávio Danni Fuchs and Sandra Costa Fuchs, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Rio Grande do Sul, provided aggregate results of participants without previous antihypertensive treatment for the study PREVER‐treatment 2016.
Yuan Jinqiu, School of Public Health and Primary Care, The Chinese University of Hong Kong, checked the inclusion criteria of an article written in Chinese.
Kateryna Kuzmytska Kalayda, general practitioner, Navarre Health Service, Tafalla, Spain, checked the inclusion criteria of an article written in Russian.
Agustín Ciapponi and Demian Glujovsky, Institute of Clinical Effectiveness and Health Policy, Buenos Aires, Argentina, provided access to EROS.
Miguel Angel Imízcoz, retired cardiologist at Navarre Health Service, Pamplona, Spain, helped with the assessment of cardiovascular events reported in studies.
José J Elizondo, hospital pharmacist at CHN‐B, Navarre Health Service, Pamplona, Spain, contributed as author to a previous version of this systematic review.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to March 10, 2021> Search Date: 11 March 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp thiazides/
2 exp sodium chloride symporter inhibitors/
3 exp sodium potassium chloride symporter inhibitors/
4 thiazide*.tw,kf.
5 ((sodium chloride adj2 cotransporter inhibit*) or (sodium chloride adj2 co‐transporter inhibit*) or (sodium chloride adj2 symporter inhibit*)).tw,kf.
6 ((ceiling adj2 diuretic*) or (loop adj2 diuretic*) or (potassium‐depleting adj2 diuretic*)).tw,kf.
7 amiloride/ or (amiloride or amiclaran or amidal or amiduret trom or amikal or amiloberag or amilorid or amiloridehydrochlorhydrate or amiloridine or amipramidine or amyloride or arumil or berkamil or colectril or guanamprazine or kaluril or medamor or midamor or midoride or modamide or nirulid or pandiuren).tw,kf.
8 (azosemide or azosemid or luret).mp.
9 bendroflumethiazide/ or (bendroflumethiazide or aprinox or bendrofluazide or bendroflumethiazide or benzhydroflumethiazide or benzydroflumethiazide or benzyl hydroflumethiazide or benzylhydroflumethiazide or benzide or berkozide or bristuron or centonuron or centyl or esberizid or naturetin or naturine or neo naclex or neonaclex or naturetin or naturine or neonadex or pluryl or pluryle or repicin or salures or sinesalin or urizid).tw,kf.
10 benzothiadiazine*.mp.
11 bumetanide/ or (bumetanide or budema or bumedyl or bumelex or bumet or bumetamide or bumethanide or bumetidine or bumex or burinax or burinex or busix or butinat or butinon or bymex or cambiex or drenural or farmadiuril or fontego or fordiuran or lixil or lunetoron or miccil or primex).tw,kf.
12 (butizide or buthiazide or eunephran or eunepran or isobutylhydrochlorothiazide or modenol or saltucin or thiabulazid or thiabutazide or thiobulazid or tiabutazide).mp.
13 chlorothiazide/ or (chlorothiazide or chlorosal or chlorothiazid or chlorothiazidum or chlorothiazine or chlorthiazide or chlotride or diachlor or diuril or diurilix or diuriwas wassermann milano or flumen or lyovac or saluric or warduzuide).tw,kf.
14 chlorthalidone/ or (chlorthalidone or aquadon or chlorphthalidolone or chlortalidon or chlortalidone or clortalidone or chlorthalidine or chlorthalidon or chlorthialidone or clortalil or edemdal or hidronal or higroton or higrotona or hygroton or hylidone or hypertol or hythalton or igrolina or igroton or isoren or natriuran or oxodolin or oxodoline or phthalamidine or phthalamodine or phthalamudine or renon or servidone or thalitone or urandil or urofinil or zambesil).tw,kf.
15 (cicletanine or cicletanide or cycletanide or justar or tenstaten or tenstatin).mp.
16 clopamide/ or (clopamide or adurix or aquez or brinaldix or brinaldrix or brinedine or chlosudimeprimylum clopamid or clopamidum or clopamine).tw,kf.
17 clorexolone/ or (clorexolone or anhydron or clorexone or chlorexolone or cyclothizaide or doburil or flonatril or fluidil or klorex or nefrolan or valmiran).tw,kf.
18 cyclopenthiazide/ or (cyclopenthiazide or cyclomethiazide or cyclopenthiazine or cyclopentiazide or navidex or navidrex or navidrix or salimid or tsiklometiazid).tw,kf.
19 ethacrynic acid/ or (ethacrynic acid or edecril or edecrin or edecrina or endecril or etacrinic acid or etacrynate or etacrynic acid or ethacrinic acid or ethacrynate or ethacryonic acid or ethocrynic acid or ethycrynic acid or hydromedin or lyovac sodium edecrin or reomax or sodium ethacrynate or uregit or uregyt).tw,kf.
20 eplerenone/ or (eplerenone or elecor or eplerenon or epoxymexrenone or inspra).tw,kf.
21 (etozolin or elkapin or etazolin or etozoline or ozolinone ethyl ester).mp.
22 (fenquizone or idrolone).mp.
23 furosemide/ or (furosemide or aldic or aluzine or anfuramaide or aquarid or arasemide or cetasix or desal or diamazon or dirine or discoid or diumide or diural or diuresal or diurin or diurix or diurolasa or diusemide or diuspec or dryptal or durafurid or edenol or errolon or eutensin or eutensine or flurosemide or franyl or fretic or frumid or frusedan or frusehexal or frusema or frusemidor frusemide or frusid or fruzex or fumarenid or fumide or furanthril or furantral or furantril or furanturil or furasemide or furesin or furesis or furetic or furix or furmid or furo puren or furo‐basan or furo‐puren or furobasan or furomen or furomex or furomide or furomin or furopuren or furorese or furosamide or furoscan or furose or furosemid or furosemix or furosimide or furosix or furovite or fursemide or fusid or fusimex or hissuflux or hydro rapid or impugan or jufurix or kofuzon or kutrix or lasiletten or lasilix or lasix or laxis or laxur or luramide or marsemide or mirfat or odemase or odemex or oedemase or oedemex or pharmix or promedes or radisemide or rasitol or retep or salinex or seguril or selectofur or sigasalur or uremide or uresix or urex‐m or vesix or zafurida).tw,kf.
24 hydrochlorothiazide/ or (hydrochlorothiazide or apo‐hydro or aquarius or aquazide or bisalunil or bpzide or bremil or chlorosulthiadil or chlorsulfonamidodihydrobenzothiadiazine or cidrex or clothia or dehydratin or diaqua or dichlorosal or dichlothiazide or dichlotride or dichlozid or diclotride or didralin or dihydrochlorothiazide or dihydrodiuril or direma or disaluril or disothiazide or dithiazide or diu melusin or diumelusin or diurace or diurex or esidrex or esidrix or fluvin or hctz or hidrenox or hidril or hidroronol or hidrosaluretil or hudorex or hychlozide or hydrex‐semi or hydril or hydro aquil or hydrochlor or hydrochloro thiazide or hydrochlorothiamide or hydrochlorothiazid or hydrochlorothiazine or hydrochlorzide or hydrochlothiazide or hydro diuril or hydrodiuril or hydromal or hydrororonol or hydro saluric or hydrosaluric or hydrothide or hydro tonuron or hydrozide or hypothiazid or hypothiazide or ivaugan or maschitt or microzide or mictrin or nefrix or neoflumen or newtolide or niagar or oretic or pantemon or ridaq or sectrazide or tandiur or thiadril or thiaretic or thiuretic or urodiazin or urodiazine or urozide or vetidrex).tw,kf.
25 hydroflumethiazide/ or (hydroflumethiazide or bristab or di ademil or diademil or dihydroflumethiazide or diraudixin or diucardin or hiserpin or hydrenox or leodrin or leodrine or metflorylthiadiazine or naclex or rontyl or saluron or sisuril or trifluoromethylhydrothiazide).tw,kf.
26 indapamide/ or (indapamide or agelan or apadex or arifon or damide or dapamax or diflerix or dixamid or extur or fludex or fluidema or frumeron or indahexal or indalix or indamol or indapam or indapress or indicontin or indoline or indopamide or inpamide or insig or ipamix or lorvas or loxide or lozol or metindamide or millibar or naplin or natrilix or natrix or noranat or pamid or pressural or pretanix or rinalix or sicco or tandix or tertensif or veroxil).tw,kf.
27 (indacrinone or indacrinic acid or indacrynic acid).mp.
28 mefruside/ or (mefruside or bay caron or baycaron or baycarone or mefrusid).tw,kf.
29 metolazone/ or (metolazone or barolyn or diulo or metalazone or metenix or metolazon or miclox or microx or mykrox or normelan or xuret or zaroxolyn).tw,kf.
30 methyclothiazide/ or (methylclothiazide or aquatensen or enduron or enduron‐m or enduronum or methyclothiazide or methylchlorothiazide or thiazidil).tw,kf.
31 muzolimine/ or (muzolimine or edrul or musolimino).tw,kf.
32 ozolinone.mp.
33 phenoxybenzoic acid.mp.
34 (piretanide or arelix or arlix or eurelix or lafax or perbilen or tauliz).mp.
35 polythiazide/ or (polythiazide or drenusil or nephril or polythiazide or renese).tw,kf.
36 (quinethazone or aquamox or chinethazon or chinethazone or guinethazone or hydromox or kinetazone or quinethazon).mp.
37 spironolactone/ or (spironolactone or abbolactone or acelat or adultmin or alaton or alatone or aldace or aldactone or aldopur or aldospirone or almatol or aquareduct or berlactone or diram or duraspiron or dyta urese or dytaurese or espironolactona or flumach or frumikal or hypazon or idrolattone or jenaspiron or merabis or novospiroton or osiren or osyrol or pirolacton or pondactone or practon or resacton or spiractin or spiridon or spirix or spiro or spiroctan or spirobeta or spirogamma or spirolacton or spirolactone or spirolang or spiron or spirone or spironex or spirono or spironol or spironone or spirospare or spirothiobarbiturate or spirotone or supra puren or suprapuren or uractone or veroshpiron or verospiron or verospirone or xenalon or youlactone).tw,kf.
38 (tiamizide or diapamide or thiamizide).mp.
39 ticrynafen/ or (ticrynafen or diflurex or selacryn or selacryn or thienilic acid or thienylic acid or tienilic acid).tw,kf.
40 tizolemide.mp.
41 torsemide/ or (torsemide or demadex or dilutol or diuremid or isemid or isodiur or luprac or presaril or sutril or toradiur or torem or torrem or torasemide or unat or upcard).tw,kf.
42 triamterene/ or (triamterene or dyrenium or dytac or urocaudal or ademin or ademine or dyren or dyrenium or dytac or iatropur or jatropur or noridyl or pterofen or pterophene or teriam or triampterene or triamterence or triamterens or triamteril or triteren or uretren or urocaudal).tw,kf.
43 trichloromethiazide/ or (trichloromethiazide or aquazide or dichloromethylhydrochlorothiazide or diurese or esmarin or eurinol or fluitran or flutra or gangesol or hydrotrichlorothiazide or metahydrin or methahydrin or naqua or naquasone or salurin or triazide or trichlordiuride or trichlorex or trichlormethazide or trichlormethiazide or trichlormas or trichloromethylhydrochlorothiazide or triflumen or wadel).tw,kf.
44 (tripamide or normonal).mp.
45 xipamide/ or (xipamide or aquaforil or aquaphor or aquaphoril or aquavor or diurexan or lumitens or xipamid or xypamide or zipix).tw,kf.
46 or/1‐45
47 exp angiotensin‐converting enzyme inhibitors/
48 ((angiotensin converting enzyme adj2 antagonist*) or (angiotensin I‐converting enzyme adj2 antagonist*)).tw,kf.
49 ((angiotensin converting enzyme adj2 inhibit*) or (angiotensin I‐converting enzyme adj2 inhibit*)).tw,kf.
50 (ace adj2 inhibit*).tw,kf.
51 (acei or aceis).tw,kf.
52 (alacepril or alazapril or cetapril).mp.
53 (altiopril or lowpress).mp.
54 (benazepril or benace or boncordin or briem or brien or cibace or cibacen or cibacene or fortekor or lotensin or tenkuoren or zinadril).mp.
55 captopril/ or (captopril or ace‐bloc or acenorm or acepress or acepril or aceprilex or aceril or aceten or adocor or alopresin or altran or apuzin or asisten or capace or capocard or caposan or capoten or capotena or capotril or capril or captace or captensin or capti or captoflux or captohexal or captolane or captomax or capton or captopren or captoprilan or captoril or captral or cardiopril or cardipril or catona or catoplin or catopril or cesplon or cryopril or debax or dexacap or dextro or ecapres or ecaten or epicordin or epsitron or farcopril or farmoten or hiperil or hypopress or hypotensor or insucar or iopril or isopresol or katopil or ketanine or keyerpril or lapril or locap or lopirin or lopril or medepres or midrat or minitent or nolectin or oltens or petacilon or praten or primace or proline or ropril or smarten or tenofax or tensicap or tensiomen or tensiomin or tensobon or tensoprel or tensoril or tenzib or topace or toprilem or typril‐ace or vasosta or zapto or zorkaptil).tw,kf.
56 (ceronapril or novopril).mp.
57 cilazapril/ or (cilazapril or cilazipril or cilazapril or dynorm or inhibace or inibace or initiss or inocar or justor or vascace or vascase).tw,kf.
58 (delapril or adecut or alindapril or deacetylalacepril or derapril).mp.
59 enalapril/ or (enalapril or renitec or renitek).tw,kf.
60 enalaprilat/ or (enalaprilat or enalaprilic or Vasotec or xanef).tw,kf.
61 (epicaptopril or fasidotril or alatriopril).mp.
62 fosinopril/ or (fosinopril or acenor‐m or bpnorm or dynacil or eliten or fosenopril or fosinil or fosinonorm or fosinorm or fosipres or fositen or fositens or fovas or fozitec or hiperlex or monopril or newace or sapril or staril or tenso stop or tensocardil or vasopril).tw,kf.
63 (foroxymithine or gemopatrilat or libenzapril or idapril or imidapril or novarok or tanapril or tanatril or Indolapril or indalapril).mp.
64 lisinopril/ or (lisinopril or acerbon or alapril or alfaken or carace or cipril or coric or dapril or fibsol or inopril or linopril or linvas or lipril or lisi abz or lisibeta or lisigamma or lisihexal or lisipril or lisodur or lisopress or lisopril or lisoril or lispril or listril or lysinopril or noperten or novatec or presiten or prinil or prinivil or qbrelis or sinopril or tensopril or tensyn or vivatec or zestomax or zestril).tw,kf.
65 (moexipril or fampress or femipres or fempres or fempress or frempress or moex or perdix or primoxil or tensotec or univasc or univase).mp.
66 (moveltipril or pentopril).mp.
67 (omapatrilat or vanlev or pentropril or pivopril or pivalopril).mp.
68 perindopril/ or (perindopril or armix arginin or bioprexanil or coverex or coversoral or coversum or coversyl or mariper or perindoprilarginin or perineva or perstarium or pirindopril or prestarium or prexanil or procaptan).tw,kf.
69 quinapril/ or (quinapril or accupril or accuprin or accupro or accupron or acequin or acuitel or acuprel or acupril or asig or conan or ectren or korec or quinalapril or quinaten or quinazil or quinhexal or quinipril).tw,kf.
70 ramipril/ or (ramipril or acovil or altace or carasel or cardace or corpril or delix or hypren or hytren or lostapres or pramace or ramace or ramilich or triatec or tritace or unipril or vesdil or vivace or zabien).tw,kf.
71 (rentiapril or fentiapril or pentiapril or s‐nitrosocaptopril or temocapril).mp.
72 (spirapril or equaten or quadropil or quadropril or renormax or renpress or sandopril or wandopres).mp.
73 (temocapril or acecol).mp.
74 teprotide/ or (teprotide or bradykinin potentiating or nonapeptide converting enzyme inhibitor* or pyroglutamyltryptophylprolylarginylprolylglutaminylisoleucylprolylproline).tw,kf.
75 (trandolapril or gopten or mavik or odace or odric or odrik or udrik or utibapril or zabicipril or zofenopril or bifril or zofenil or zofenoprilum or zopranol).mp.
76 or/47‐75
77 exp Angiotensin Receptor Antagonists/
78 (angiotensin adj3 receptor antagon*).tw,kf.
79 (angiotensin adj3 receptor block*).tw,kf.
80 (arb or arbs).tw,kf.
81 (sartan or sartans).tw,kf.
82 (azilsartan or edarbi or ipreziv).mp
83 candesartan.mp.
84 (elisartan or embusartan or forasartan or milfasartan or pratosartan or KT3‐671 or saprisartan or zolasartan).mp.
85 (eprosartan or epratenz or futuran or naviten or navixen or regulaten or tevesten or tevetan or teveten or tevetenz).mp.
86 irbesartan/ or (irbesartan or approvel or aprovel or arbez lr or avapro or ifirmasta or Irban or irbetan or iretensa or irovel or irvell or karvea).tw,kf.
87 losartan/ or (losartan or cozaar).tw,kf.
88 olmesartan medoxomil/ or (olmesartan or alteis or belsar or benetor or benevas or benicar or ixia or laresin or mencord or mesar or olartan or olmeblo or olmec or olmes or olmetec or olpress or olsar or omesar or openvas or plaunac or santini or sarten or tensar or tensiol or vivactra or votum).tw,kf.
89 (tasosartan or anazor or verdia).mp.
90 telmisartan/ or (telmisartan or actelsar or "kinzal mono" or kinzalmono or micardis or predxal or pritor or pritoral or semintra or tolura).tw,kf.
91 valsartan/ or (valsartan or angiosan or cordinate or dalzad or diovan or diovane or kalpress or miten or nisis or prexxartan or provas or rixil or saval or tareg or tazea or troval or valpression or vals or valsocard or valtan or valtsu).tw,kf.
92 or/77‐91
93 renin/ai
94 (RAS adj2 inhibit*).tw,kf.
95 (renin adj2 inhibit*).tw,kf.
96 (aliskiren or enviage or rasilez or riprazo or sprimeo or tekturna).mp.
97 ciprokiren.mp.
98 remikiren.mp.
99 terlakiren.mp.
100 zankiren.mp.
101 or/93‐100
102 exp calcium channel blockers/
103 ((calcium adj2 antagonist*) or (calcium adj2 block*) or (calcium adj3 inhibit*)).tw,kf.
104 exp amlodipine/ or (amlodipine or amdip or amloc or amlodipin or amlodipina or amlodis or amlopin or amlor or astudal or istin or levamlodipine or norvasc).tw,kf.
105 amrinone/ or (amrinone or amcoral or amrinone or cardiotone or cartonic or cordemcura or inamrinone or inocor or vesistol or wincoram).tw,kf.
106 (aranidipine or bec or sapresta).mp.
107 bencyclane/ or (bencyclane or bencyclan or benzcyclan or benzyclane or desoblit or dilangio compositu or fludilat or fluxema or halidor or ludilat).tw,kf.
108 (benidipine or coniel).mp.
109 bepridil/ or (bepridil or angopril or bedapin or bepadin or bepricol or cordium or cruor or unicordium or vascor).mp.
110 (cilnidipine or atelec or cinaldipine or cinalong or siscard).mp.
111 cinnarizine/ or (cinnarizine or aplactan or aplexal or apomitere or apotomin or artate or carecin or cerebolan or cerepar or cibine or cimarizine or cinabioquim or cinaperazine or cinarizina or cinarizine or cinaziere or cinazyn or cinna or cinnabene or cinnacet or cinnaforte or cinnageron or cinnarazine or cinnarizin or cinnipirine or cinniprine or cisaken or corathiem or denapol or dimitron or dimitronal or eglen or giganten or glanil or hilactan or ixertol or katoseran or labyrin or lazeta or marisan or midronal or mitronal or olamin or processine or roin or sedatromin or sepan or siptazin or spaderizine or stugeron or stutgeron or stutgin).tw,kf.
112 (clentiazem or logna or darodipine or dazodipine).mp.
113 diltiazem/ or (diltiazem or acalix or adizeml or anoheal or anotrit or anzem or au or aldizem or altiazem or anginyl or angiodrox or angiotrofen or angiotrofin or angiozem or angitil or angizem or angorascard or balcor or beatizem or blocalcin or britiazim or bruzem or calcicard or calnurs or cardcal or cardiazem or cardiben or cardiem or cardil or cardiosta or cardium or cardizem or carex or carreldon or cartia xt or cascor xl or cirilen or coras or cordizem or dazil or deltazen or diacor or diatal or diazem or dilacor or diladel or dilatam or dilatame or dilcard or dilcardia or dilem or dilfar or dilgard or diloc or dilren or dilrene or dilso or dilt‐cd or diltahexal or diltam or diltan or diltelan or diltia or diltiamax or diltiasyn or diltime or diltiwas or diltzac or dilzanton or dilzem or dilzene or dilzereal or dilzicardin or dinisor or doclis or dodexen or dyalac or entrydil or etizem or filazem or gadoserin or grifodilzem or hagen or helsibon or herben or herbesser or hesor or incoril or kaizem or kenzem or lacerol or levodex or levozem or lytelsen or masdil or miocardie or monotildiem or myonil or pazeadin or presoken or slozem or surazem or tazem or taztia or tiadil or tiamate or tiazac or tilazem or tildiem or tilker or vasmulax or vasocardol or wentizem or zandil or zatim or zemret or zemtrial or zildem or ziruvate).tw,kf.
114 (efonidipine or finte or landel or elgodipine).mp.
115 (etafenone or baxacor or dialicor or diethylaminoethoxyphenylpropiophenone hydrochloride or etafenon or etaphenone or kca or revisor).mp.
116 fantofarone.mp.
117 felodipine/ or (felodipine or agon or dilahex or dilofen or dilopin or fedil or felim or felo biochemie or felo er or felo‐basf or felo puren or felobal or felobeta or felocor or felodipin or felodur or felogamma or felogard or felop or felopine‐sr or fensel or flodil or h15482 or hydac or keydipin or lodistad or modip or munobal or nirmadil or penedil or perfudal or plendil or preslow or prevex or renedil or selepine or splendil or versant xr).tw,kf.
118 fendiline/ or (fendiline or cordan or digosensit or fendilar or fendiline or phendiline or sensit or senzit).tw,kf.
119 flunarizine/ or (flunarizine or flunarizine or sibelium).tw,kf.
120 gallopamil/ or (gallopamil or elgiprona or galcan or gallobeta or gallopamile or methoxyverapamil or prebet or procorum).tw,kf.
121 isradipine/ or (isradipine or dynacirc or icaz or isrodipine or lomir or prescal or vascal).tw,kf.
122 (lacidipine or aponil or caldine or lacimen or lacipil or lacirex or lacitens or motens or viapres).mp.
123 (lercanidipine or carmen or corifeo or lercadip or lercan or lerdip or masnidipine or zanedip or zanidip).mp.
124 lidoflazine/ or (lidoflazine or clinium or ordiflazine).tw,kf.
125 (lomerizine or migsis or terranas).mp.
126 (manidipine or artedil or calslot or franidipine or iperten or manyper or vascoman).mp.
127 (mepirodipine or barnidipine or cyress or hypoca or libradin or vasexten).mp.
128 mibefradil/ or (mibefradil or posicor).tw,kf.
129 nicardipine/ or (nicardipine or antagonil or barizine or bionicard or cardene or cardepine or cardibloc or cardipene or dacarel or dagan or flusemide or karden or lecibral or lincil or loxen or lucenfal or nerdipine or nicardal or nicardil or nicardipino or nicarpin or nicodel or nimicor or perdipina or perdipine or ranvil or ridene or roxen or rycarden or rydene or saf card or vasodin or vasonase).tw,kf.
130 nifedipine/ or (nifedipine or adalat or cordipin or cordipine or corinfar or fenigidin or korinfar or nifangin or procardia or vascard).tw,kf.
131 niguldipine.mp.
132 (nilvadipine or escor or nilvadil or nivadil or nivadipine).tw,kf.
133 nimodipine/ or (nimodipine or admon or brainal or calnit or eugerial or grifonimod or kenesil or kenzolol or modus or nidip or nimodilat or nimodipin or nimodipin‐isis or nimodipino or nimotop or nisom or nymalize or periplum or remontal or tropocer or vasoflex or vasotop).tw,kf.
134 (nisoldipine or angiolat or baymycard or corasol or nisoldin or sular or syscor).mp.
135 nitrendipine/ or (nitrendipine or balminil or baylotensin or bayotensin or baypresol or baypress or gericin or jutapress or lusopress or nidrel or niprina or nitre or nitregamma or nitren or nitrend ksk or nitrepress or nitre‐puren or nitrepuren or nitrendepat or nitrendi biochemie or nitrendidoc or nitrendimerck or nitrendipin or nitrendipin‐corax or nitrendipin‐ratiopharm or nitrendipincorax or nitrendipino or nitrendipinratiopharm or nitrensal or tensogradal or trendinol or vastensium).tw,kf.
136 perhexiline/ or (perhexilene or perhexiline).tw,kf.
137 prenylamine/ or (prenylamine or corontin or difril or segontin).tw,kf.
138 (semotiadil or levosemotiadil or sesamodil).mp.
139 (terodiline or biro or mictrol or micturin or terrain).mp.
140 tiapamil hydrochloride/ or (tiapamil or dimeditiapramine or larocord or thiapamil or verocainide or verocainine).tw,kf.
141 verapamil/ or (verapamil or apo‐verap or apoacor or arpamyl or azupamil or berkatens or calan or calaptin or cardiagutt or cardibeltin or cardiolen or cardiovert or caveril or cintsu or civicor or coraver or cordilat or cordilox or corpamil or covera or dexverapamil or dignover or dilacoran or dilacoron or durasoptin or falicard or finoptin or flamon or geangin or hexasoptin or ikacor or ikakor or ikapress or iproveratril or iso‐card sr or isoptin or isoptine or isoptino or izoptin or lekoptin or manidon or napamil or novapamyl or novo veramil or novopressan or phynoptin or quasar or ravamil or securon or univer or vasolan or vasomil or vasopten or verabeta or veracaps sr or veracor or verahexal or veraloc or veramex or veramil or verapin or verapress or veratad or verdilac or verelan or verexamil or veroptin stada or verpamil or vetrimil or vortac or zolvera).tw,kf.
142 or/102‐141
143 exp adrenergic beta‐antagonists
144 ((beta adj2 adrenergic*) or (beta adj2 antagonist*)).tw,kf.
145 ((beta adj2 block*) or (beta adj2 receptor*)).tw,kf.
146 acebutolol/ or (acebutolol or acecor or acetobutolol or apoacebutolol or diasectral or espesil or flebutol or grifobutol or monitan or neptal or neptall or novoacebutolol or prent or rhotral or sectral).tw,kf.
147 alprenolol/ or (alprenolol or alfeprol or alloprenalol or alpheprol or alprendol or alprenol or alprenololum or apliobal or aprenolol or aptia or aptin or aptin‐duriles or aptinduriles or aptina or aptine or aptol or astra or betacard or betapin).tw,kf.
148 (amosulalol or amsolulol or howgan or lowgan).mp.
149 (arotinolol or almarl).mp.
150 atenolol/ or (atenolol or ablok or adoll or alonet or altol or anolene or anolpin or anselol or arandin or asten or atarox or atcardil or atecard or atehexal or atelol or atenblock or atendol or atenet or ateni or atenil or ateno or atenogamma or atenol or atereal or aterol or atestad or atinol or atolmin or b‐vasc or betablok or betacar or betarol or betatop ge or beten or bloket or blokium or blotex or cardioten or catenol or coratol or corotenol or durabeta or esatenolol or evitocor or farnormin or felo‐bits or hypernol or hypoten or internolol or lo‐ten or loten or lotenal or martenol or mirobect or myocord or neotenol or nolol or normalol or normaten or normiten or nortelol or noten or oraday or ormidol or paesumex or plenacor or preloc or premorine or prenolol or prenormine or ranlol or rozamin or serten or stermin or temoret or tenblok or tenidon or tenoblock or tenocor or tenol or tenolin or tenolol or tenopress or tenoprin or tenormin or tenormine or tenostat or tensig or tensinor or ternolol or therabloc or tredol or vascoten or velorin or vericordin or wesipin).tw,kf.
151 (befunolol or befnolol or benfuran or bentos or bentox or glauconex or glaukonex).mp.
152 betaxolol/ or (betaxolol or "alcon betoptic" or betac or betarun or betasel or betaxon or betoptic or betoptima or betoquin or kerlon or kerlone or kerlong or levobetaxolol or lokren or optibet or optipress or oxodal or tonobexol).tw,kf.
153 (bevantolol or benatol or bevantololum or calvan or ranestol or vantol).mp.
154 bisoprolol/ or (bisoprolol or concor).tw,kf.
155 (bopindolol or sandonorm or wandonorm).mp.
156 bucindolol.mp.
157 (bucumolol or bucumarol or bucumolol).mp.
158 (bufarolol or angium).mp.
159 (bufetolol or adbiol).mp.
160 (bunitrolol or betrilol or stresson or tilcant).mp.
161 butofilolol.mp.
162 (carazolol or conducton or suacron).mp.
163 carteolol/ or (carteolol or arteolol or arteoptic or arteoptik or caltamol or calte or carbonolol or carteabak or carteol or cartrol or elebloc or endak or glauteolol or karol or karteol or mikelan or ocupress or stobol or teoptic).tw,kf.
164 carvedilol/ or (carvedilol or cardiol or cardivas or carvedlol or carvipress or carvrol or coreg or dilatrend or dilbloc or dimitone or eucardic or kredex or quarto or quarto or v‐bloc).tw,kf.
165 celiprolol/ or (celiprolol or abecor or celectol or dilanorm or selecor or selectol).tw,kf.
166 (cetamolol or betacor).mp.
167 (cloranolol or tobanum).mp.
168 (epanolol or visacor).mp.
169 (esmolol or brevibloc or miniblock).mp.
170 (indenolol or pulsan).mp.
171 labetolol/ or (labetalol or abetol or albetol or amipress or apolabetalol or biascor or dilevalol or dilevalon or hybloc or ibidomide or ipolab or labelol or labesine or lamitol or levadil or liondox or normodyne or presdate or presolol or pressalolo or salmagne or trandate or unicard).tw,kf.
172 levobunolol/ or (levobunolol or "ak beta" or akbeta or apolevobunolol or betagan or betasite or bunolgan or gotensin or l bunolol hydrochloride or levo bunolol or novolevobunolol or pmslevobunolol or ultracortenol or vistagan or vistagen).tw,kf.
173 (mepindolol or betagon or caridian or corindolan).mp.
174 metipranolol/ or (metipranolol or beta ophtiole or betalol or betamann or betamet or betanol or betanolol or disorat or glauline or methypranol or normoglaucon mite or ophtiole or optipranolol or trimepranol).tw,kf.
175 metoprolol/ or (metoprolol or beloc duriles or belok zok or betaloc or betalok or lopressor or seloken or spesicor or spesikor or toprol).tw,kf.
176 moprolol.mp.
177 nadolol/ or (nadolol or betadol or corgard or farmagard or nadic or solgol or solgol).tw,kf.
178 nadoxolol.mp.
179 nebivolol/ or (nebivolol or bivolet or bystolic or dexnebivolol or levonebivolol or lobivon or narbivolol or nebilet or nebilox or silostar).tw,kf.
180 (nifenalol or "d inpea" or "dextro inpea" or dextro levo inpea or inpea or isophenethanol or isophenethyl alcohol or isopropylaminonitrophenylethanol or nefenalol).mp.
181 oxprenolol/ or (oxprenolol or cordexol or coretal or koretal or oxtrenolol or tevacor or trasacor or trasicor).tw,kf.
182 pafenolol.mp.
183 penbutolol/ or (penbutolol or betapresin or betapressin or blocotin or levatolor paginol).tw,kf.
184 pindolol/ or (pindolol or barbloc or betapindol or blocklin or calvisken or carvisken or decreten or dranolis or durapindol or hexapindol or hydroxypropylaminopropoxyindol or nonspi or novo‐pindol or pectobloc or pectoblock mite or pidol or pinbetol or pinden or pindol or pindomex or pindoreal or pinloc or pinolol or pinsken or prindolol or prinodolol or pyndale or treparasen or viskeen or visken or viskene or vypen).tw,kf.
185 practolol/ or (practolol or dalzic or eraldin or practolole or praktolol or praktololu or proctalol or teranol).tw,kf.
186 (pronethalol or alderlin or nethalide or pronetal or pronetalol).mp.
187 propanolol/ or (propranolol or acifol or adrexan or alperol or anaprilin or anapriline or anaprilinium or anapryline or angilol or apsolol or arcablock or artensol or authus or avlocardyl or becardin or bedranol or beprane or bercolol or berkolol or beta neg or beta tablinen or beta timelets or betabloc or betadipresan or betadren or betaneg or betaprol or betares or betaryl or blocard or blocaryl or cardinol or ciplar or corbeta or deralin or dexpropranolol or dibudinate or dideral or dociton or docitone or durabeton or duranol or efektolol or elbrol or emforal or farmadral or farprolol or frekven or frina or hemangeol or hemangiol or hopranolol or ikopal or Impral or Inderal or inderalici or inderex or indicardin or indobloc or innopran or inpanol or ipran or lederpronol or levopropranolol or napriline or noloten or obsidan or obsin or obzidan or oposim or phanerol or prandol or prano puren or pranopuren or prestoral or prolol or pronovan or propabloc or propal or propalong or propayerst or propercuten or prophylux or propra ratiopharm or propral or propranur or proprasylyt or proprasylyte or reducor or rexigen or sagittol or stapranolol or sumial or tenomal or tensiflex or waucoton).tw,kf.
188 sotalol/ or (sotalol or alosot or beta‐cardone or betacardone or betades or betapace or bonpro or corsotalol or darob or dexsotalol or dextrosotalol or favorex or gilucor or hipecor or isotalol or jutalex or levosotalol or rentibloc or rotalol or satalol or satolol or solavert or sorine or sota saar or sotab or sotabeta or sotacol or sotacor or sotahexal or sotalex or sotaper or sotapor or sotastad or sotylize or tachytalol).tw,kf.
189 (sulfinalol or talinolol or cordanum).mp.
190 (tertatolol or artex or prenalex).mp.
191 (tilisolol or daim or selecal).mp.
192 timolol/ or (timolol or apotimolol or blocadren or optimal or timacar or timolo or timoptic or timoptol or titol).tw,kf.
193 (toliprolol or doberol).mp.
194 xibenolol.mp.
195 or/143‐194
196 exp adrenergic alpha antagonists/
197 ((adrenergic adj2 alpha) or (adrenergic adj2 antagonist*)).tw,kf.
198 ((adrenergic adj2 block*) or (alpha adj2 block*) or (receptor* adj2 block*)).tw,kf.
199 (alpha adj2 receptor antagonist*).tw,kf.
200 (alfuzosin or bunazosin or metazosin or neldazosin or silodosin or terazosin or tiodazosin or trimazosin).mp.
201 doxazosin/ or (doxazosin* or alfamedin or cardular or cardura or carduran or diblocin or doxa puren or doxauro or doxacor or doxagamma or doxamax or doxatensa or doxazomerck or doxazosina or jutalar or progandol or uriduct or uroprost or zoxan).tw,kf.
202 prazosin/ or (prazosin or adversuten or alpress or atodel or decliten or deprazolin or duramipress or eurex or furazosin or hexapress or hypotens or hypovase or hyprosin or lentopres or minipres or minesin or minipres or minipress or minison or mizosin or patsolin or peripress or prasig or pratisol or pratsiol or prazac or prazopress or prazosine or pressin or prozosin).tw,kf.
203 tamsulosin/ or (tamsulosin or alna or amsulosin or flomax or flomaxtra or hamal or josir or mapelor or mecir or omexel or omic or omix or omnic or pradif or secotex or tamfrex or urolosin).tw,kf.
204 or/196‐203
205 (46 and 76) or (46 and 92) or (46 and 101) or (46 and 142) or (46 and 195) or (46 and 204) or (76 and 92) or (76 and 101) or (76 and 142) or (76 and 195) or (76 and 204) or (92 and 101) or (92 and 142) or (92 and 195) or (92 and 204) or (101 and 142) or (101 and 195) or (101 and 204) or (142 and 195) or (142 and 204) or (195 and 204)
206 drug therapy, combination/
207 (add* or combin* or multipl* or plus or polytherap* or versus or vs).tw,kf,kw,ot.
208 or/206‐207
209 hypertension/
210 essential hypertension/
211 hypertens*.tw,kf.
212 ((chang* or elevat* or high or lower* or rais*) adj2 (arterial pressur* or blood pressur* or diastolic pressur* or systolic pressur*)).tw,kf.
213 ((chang* or elevat* or high or lower* or rais*) adj2 (bp or dbp or sbp)).tw,kf.
214 or/209‐213
215 randomized controlled trial.pt.
216 controlled clinical trial.pt.
217 randomi*ed.ab.
218 placebo.ab.
219 dt.fs.
220 randomly.ab.
221 trial.ab.
222 groups.ab.
223 or/215‐222
224 animals/ not (humans/ and animals/)
225 exp Hypertension, Pregnancy‐Induced/ or Hypertension, Pulmonary/ or Hypertension, Portal/ or Hypertension, Renovascular/ or exp *Obstetrics/ or exp Ocular Hypertension/ or exp *Pregnancy/ or exp *Pregnancy Complications/ or exp *Prenatal Education/
226 (anaesth* or anesth* or ante natal* or antenatal* or bleeding or caesarean or cesarean or caesarian or cesarian or caesarien or cesarien or cirrho* or eclamp* or epidural or fetal or foetal or fetus or foetus or gestational hypertens* or glaucom* or interocular or intraocular or intracran* or intuba* or lactation* or neonat* or new born* or newborn* or obstetr* or ocular or ophthalm* or peri partum or peripartum or portal hypertens* or portopulmonary or pre eclamp* or preeclamp* or pregnancies or pregnancy or pregnant or pre natal* or prenatal* or pulmonary hypertens* or pulmonary arterial hypertens* or secondary hypertens* or spinal).ti.
227 223 not (224 or 225 or 226)
228 205 and 208 and 214 and 227
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Hypertension Group Specialised Register via Cochrane Register of Studies Search Date: 12 March 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Antihypertensive Agents EXPLODE ALL AND INREGISTER #2 antihypertens* AND INREGISTER #3 (#1 OR #2) #4 MESH DESCRIPTOR Hypertension AND INREGISTER #5 MESH DESCRIPTOR Essential Hypertension AND INREGISTER #6 hypertens* AND INREGISTER #7 ((elevat* OR high OR rais*) NEAR2 (arterial pressur* OR blood pressur* OR diastolic pressur* OR systolic pressur*)):TI,AB AND INREGISTER #8 ((elevat* OR high OR rais*) NEAR2 (bp OR dbp OR sbp)):TI,AB AND INREGISTER #9 (#4 OR #5 OR #6 OR #7 OR #8) #10 MESH DESCRIPTOR Drug Therapy, Combination EXPLODE ALL AND INREGISTER #11 (add* OR combin* OR dual OR monotherap* OR multipl* OR plus OR polytherap* OR versus OR vs) AND INREGISTER #12 (#10 OR #11) #13 #3 AND #9 AND #12 #14 RCT:DE AND INREGISTER #15 #13 AND #14 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials (Issue 2, 2021) via Cochrane Register of Studies Search Date: 11 March 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Thiazides EXPLODE ALL AND CENTRAL:TARGET #2 MESH DESCRIPTOR sodium chloride symporter inhibitors EXPLODE ALL AND CENTRAL:TARGET #3 MESH DESCRIPTOR sodium potassium chloride symporter inhibitors EXPLODE ALL AND CENTRAL:TARGET #4 thiazide*:TI,AB AND CENTRAL:TARGET #5 ((sodium chloride NEAR2 cotransporter inhibit*) OR (sodium chloride NEAR2 co‐transporter inhibit*) OR (sodium chloride NEAR2 symporter inhibit*)):TI,AB AND CENTRAL:TARGET #6 ((ceiling NEAR2 diuretic*) OR (loop NEAR2 diuretic*) OR (potassium‐depleting NEAR2 diuretic*)):TI,AB AND CENTRAL:TARGET #7 (amiloride OR amiclaran OR amidal OR amiduret trom OR amikal OR amiloberag OR amilorid OR amiloridehydrochlorhydrate OR amiloridine OR amipramidine OR amyloride OR arumil OR berkamil OR colectril OR guanamprazine OR kaluril OR medamor OR midamOR OR midoride OR modamide OR nirulid OR pandiuren):TI,AB AND CENTRAL:TARGET #8 (azosemide OR azosemid OR luret):TI,AB AND CENTRAL:TARGET #9 (bendroflumethiazide OR aprinox OR bendrofluazide OR bendroflumethiazide OR benzhydroflumethiazide OR benzydroflumethiazide OR benzyl hydroflumethiazide OR benzylhydroflumethiazide OR benzide OR berkozide OR bristuron OR centonuron OR centyl OR esberizid OR naturetin OR naturine OR neo naclex OR neonaclex OR naturetin OR naturine OR neonadex OR pluryl OR pluryle OR repicin OR salures OR sinesalin OR urizid):TI,AB AND CENTRAL:TARGET #10 benzothiadiazine*:TI,AB AND CENTRAL:TARGET #11 (bumetanide OR budema OR bumedyl OR bumelex OR bumet OR bumetamide OR bumethanide OR bumetidine OR bumex OR burinax OR burinex OR busix OR butinat OR butinon OR bymex OR cambiex OR drenural OR farmadiuril OR fontego OR fordiuran OR lixil OR lunetORon OR miccil OR primex):TI,AB AND CENTRAL:TARGET #12 (butizide OR buthiazide OR eunephran OR eunepran OR isobutylhydrochlorothiazide OR modenol OR saltucin OR thiabulazid OR thiabutazide OR thiobulazid OR tiabutazide):TI,AB AND CENTRAL:TARGET #13 (chlorothiazide OR chlorosal OR chlorothiazid OR chlorothiazidum OR chlorothiazine OR chlorthiazide OR chlotride OR diachlor OR diuril OR diurilix OR diuriwas wassermann milano OR flumen OR lyovac OR saluric OR warduzuide):TI,AB AND CENTRAL:TARGET #14 (chlorthalidone OR aquadon OR chlorphthalidolone OR chlortalidon OR chlortalidone OR clortalidone OR chlorthalidine OR chlorthalidon OR chlorthialidone OR clortalil OR edemdal OR hidronal OR higroton OR higrotona OR hygroton OR hylidone OR hypertol OR hythalton OR igrolina OR igroton OR isoren OR natriuran OR oxodolin OR oxodoline OR phthalamidine OR phthalamodine OR phthalamudine OR renon OR servidone OR thalitone OR urandil OR urofinil OR zambesil):TI,AB AND CENTRAL:TARGET #15 (cicletanine OR cicletanide OR cycletanide OR justar OR tenstaten OR tenstatin):TI,AB AND CENTRAL:TARGET #16 (clopamide OR adurix OR aquez OR brinaldix OR brinaldrix OR brinedine OR chlosudimeprimylum clopamid OR clopamidum OR clopamine):TI,AB AND CENTRAL:TARGET #17 (clorexolone OR anhydron OR clorexone OR chlorexolone OR cyclothizaide OR doburil OR flonatril OR fluidil OR klorex OR nefrolan OR valmiran):TI,AB AND CENTRAL:TARGET #18 (cyclopenthiazide OR cyclomethiazide OR cyclopenthiazine OR cyclopentiazide OR navidex OR navidrex OR navidrix OR salimid OR tsiklometiazid):TI,AB AND CENTRAL:TARGET #19 (ethacrynic acid OR edecril OR edecrin OR edecrina OR endecril OR etacrinic acid OR etacrynate OR etacrynic acid OR ethacrinic acid OR ethacrynate OR ethacryonic acid OR ethocrynic acid OR ethycrynic acid OR hydromedin OR lyovac sodium edecrin OR reomax OR sodium ethacrynate OR uregit OR uregyt):TI,AB AND CENTRAL:TARGET #20 (eplerenone OR elecor OR eplerenon OR epoxymexrenone OR inspra):TI,AB AND CENTRAL:TARGET #21 (etozolin OR elkapin OR etazolin OR etozoline OR ozolinone ethyl ester):TI,AB AND CENTRAL:TARGET #22 (fenquizone OR idrolone):TI,AB AND CENTRAL:TARGET #23 (furosemide OR aldic OR aluzine OR anfuramaide OR aquarid OR arasemide OR cetasix OR desal OR diamazon OR dirine OR discoid OR diumide OR diural OR diuresal OR diurin OR diurix OR diurolasa OR diusemide OR diuspec OR dryptal OR durafurid OR edenol OR errolon OR eutensin OR eutensine OR flurosemide OR franyl OR fretic OR frumid OR frusedan OR frusehexal OR frusema OR frusemidOR frusemide OR frusid OR fruzex OR fumarenid OR fumide OR furanthril OR furantral OR furantril OR furanturil OR furasemide OR furesin OR furesis OR furetic OR furix OR furmid OR furo puren OR furo‐basan OR furo‐puren OR furobasan OR furomen OR furomex OR furomide OR furomin OR furopuren OR furORese OR furosamide OR furoscan OR furose OR furosemid OR furosemix OR furosimide OR furosix OR furovite OR fursemide OR fusid OR fusimex OR hissuflux OR hydro rapid OR impugan OR jufurix OR kofuzon OR kutrix OR lasiletten OR lasilix OR lasix OR laxis OR laxur OR luramide OR marsemide OR mirfat OR odemase OR odemex OR oedemase OR oedemex OR pharmix OR promedes OR radisemide OR rasitol OR retep OR salinex OR seguril OR selectofur OR sigasalur OR uremide OR uresix OR urex‐m OR vesix OR zafurida):TI,AB AND CENTRAL:TARGET #24 (hydrochlorothiazide OR apo‐hydro OR aquarius OR aquazide OR bisalunil OR bpzide OR bremil OR chlorosulthiadil OR chlorsulfonamidodihydrobenzothiadiazine OR cidrex OR clothia OR dehydratin OR diaqua OR dichlorosal OR dichlothiazide OR dichlotride OR dichlozid OR diclotride OR didralin OR dihydrochlorothiazide OR dihydrodiuril OR direma OR disaluril OR disothiazide OR dithiazide OR diu melusin OR diumelusin OR diurace OR diurex OR esidrex OR esidrix OR fluvin OR hctz OR hidrenox OR hidril OR hidrORonol OR hidrosaluretil OR hudorex OR hychlozide OR hydrex‐semi OR hydril OR hydro aquil OR hydrochlor OR hydrochloro thiazide OR hydrochlorothiamide OR hydrochlorothiazid OR hydrochlorothiazine OR hydrochlorzide OR hydrochlothiazide OR hydro diuril OR hydrodiuril OR hydromal OR hydrororonol OR hydro saluric OR hydrosaluric OR hydrothide OR hydro tonuron OR hydrozide OR hypothiazid OR hypothiazide OR ivaugan OR maschitt OR microzide OR mictrin OR nefrix OR neoflumen OR newtolide OR niagar OR ORetic OR pantemon OR ridaq OR sectrazide OR tandiur OR thiadril OR thiaretic OR thiuretic OR urodiazin OR urodiazine OR urozide OR vetidrex):TI,AB AND CENTRAL:TARGET #25 (hydroflumethiazide OR bristab OR di ademil OR diademil OR dihydroflumethiazide OR diraudixin OR diucardin OR hiserpin OR hydrenox OR leodrin OR leodrine OR metflorylthiadiazine OR naclex OR rontyl OR saluron OR sisuril OR trifluoromethylhydrothiazide):TI,AB AND CENTRAL:TARGET #26 (indapamide OR agelan OR apadex OR arifon OR damide OR dapamax OR diflerix OR dixamid OR extur OR fludex OR fluidema OR frumeron OR indahexal OR indalix OR indamol OR indapam OR indapress OR indicontin OR indoline OR indopamide OR inpamide OR insig OR ipamix OR lorvas OR loxide OR lozol OR metindamide OR millibar OR naplin OR natrilix OR natrix OR noranat OR pamid OR pressural OR pretanix OR rinalix OR sicco OR tandix OR tertensif OR veroxil):TI,AB AND CENTRAL:TARGET #27 (indacrinone OR indacrinic acid OR indacrynic acid):TI,AB AND CENTRAL:TARGET #28 (mefruside OR bay caron OR baycaron OR baycarone OR mefrusid):TI,AB AND CENTRAL:TARGET #29 (metolazone OR barolyn OR diulo OR metalazone OR metenix OR metolazon OR miclox OR microx OR mykrox OR normelan OR xuret OR zaroxolyn):TI,AB AND CENTRAL:TARGET #30 (methylclothiazide OR aquatensen OR enduron OR enduron‐m OR enduronum OR methyclothiazide OR methylchlorothiazide OR thiazidil):TI,AB AND CENTRAL:TARGET #31 (muzolimine OR edrul OR musolimino):TI,AB AND CENTRAL:TARGET #32 ozolinone:TI,AB AND CENTRAL:TARGET #33 phenoxybenzoic acid:TI,AB AND CENTRAL:TARGET #34 (piretanide OR arelix OR arlix OR eurelix OR lafax OR perbilen OR tauliz):TI,AB AND CENTRAL:TARGET #35 (polythiazide OR drenusil OR nephril OR polythiazide OR renese):TI,AB AND CENTRAL:TARGET #36 (quinethazone OR aquamox OR chinethazon OR chinethazone OR guinethazone OR hydromox OR kinetazone OR quinethazon):TI,AB AND CENTRAL:TARGET #37 (spironolactone or abbolactone OR acelat OR adultmin OR alaton OR alatone OR aldace OR aldactone OR aldopur OR aldospirone OR almatol OR aquareduct OR berlactone OR diram OR duraspiron OR dyta urese OR dytaurese OR espironolactona OR flumach OR frumikal OR hypazon OR idrolattone OR jenaspiron OR merabis OR novospiroton OR osiren OR osyrol OR pirolacton OR pondactone OR practon OR resacton OR spiractin OR spiridon OR spirix OR spiro OR spiroctan OR spirobeta OR spirogamma OR spirolacton OR spirolactone OR spirolang OR spiron OR spirone OR spironex OR spirono OR spironol OR spironone OR spirospare OR spirothiobarbiturate OR spirotone OR supra puren OR suprapuren OR uractone OR veroshpiron OR verospiron OR verospirone OR xenalon OR youlactone):TI,AB AND CENTRAL:TARGET #38 (tiamizide OR diapamide OR thiamizide):TI,AB AND CENTRAL:TARGET #39 (ticrynafen OR diflurex OR selacryn OR selacryn OR thienilic acid OR thienylic acid OR tienilic acid):TI,AB AND CENTRAL:TARGET #40 tizolemide:TI,AB AND CENTRAL:TARGET #41 (torsemide OR demadex OR dilutol OR diuremid OR isemid OR isodiur OR luprac OR presaril OR sutril OR toradiur OR torem OR torrem OR torasemide OR unat OR upcard):TI,AB AND CENTRAL:TARGET #42 (triamterene OR dyrenium OR dytac OR urocaudal OR ademin OR ademine OR dyren OR dyrenium OR dytac OR iatropur OR jatropur OR noridyl OR pterofen OR pterophene OR teriam OR triampterene OR triamterence OR triamterens OR triamteril OR triteren OR uretren OR urocaudal):TI,AB AND CENTRAL:TARGET #43 (trichloromethiazide OR aquazide OR dichloromethylhydrochlorothiazide OR diurese OR esmarin OR eurinol OR fluitran OR flutra OR gangesol OR hydrotrichlorothiazide OR metahydrin OR methahydrin OR naqua OR naquasone OR salurin OR triazide OR trichlordiuride OR trichlorex OR trichlormethazide OR trichlormethiazide OR trichlormas OR trichloromethylhydrochlorothiazide OR triflumen OR wadel):TI,AB AND CENTRAL:TARGET #44 (tripamide OR normonal):TI,AB AND CENTRAL:TARGET #45 (xipamide OR aquaforil OR aquaphor OR aquaphoril OR aquavor OR diurexan OR lumitens OR xipamid OR xypamide OR zipix):TI,AB AND CENTRAL:TARGET #46 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45) #47 MESH DESCRIPTOR Angiotensin‐Converting Enzyme Inhibitors EXPLODE ALL AND CENTRAL:TARGET #48 ((angiotensin converting enzyme NEAR2 antagonist*) OR (angiotensin I‐converting enzyme NEAR2 antagonist*)):TI,AB AND CENTRAL:TARGET #49 ((angiotensin converting enzyme NEAR2 inhibit*) OR (angiotensin I‐converting enzyme NEAR2 inhibit*)):TI,AB AND CENTRAL:TARGET #50 (ace NEAR2 inhibit*):TI,AB AND CENTRAL:TARGET #51 (acei OR aceis):TI,AB AND CENTRAL:TARGET #52 (alacepril OR alazapril OR cetapril):TI,AB AND CENTRAL:TARGET #53 (altiopril OR lowpress):TI,AB AND CENTRAL:TARGET #54 (benazepril OR benace OR boncordin OR briem OR brien OR cibace OR cibacen OR cibacene OR fortekor OR lotensin OR tenkuoren OR zinadril):TI,AB AND CENTRAL:TARGET #55 (captopril OR ace‐bloc OR acenorm OR acepress OR acepril OR aceprilex OR aceril OR aceten OR adocor OR alopresin OR altran OR apuzin OR asisten OR capace OR capocard OR caposan OR capoten OR capotena OR capotril OR capril OR captace OR captensin OR capti OR captoflux OR captohexal OR captolane OR captomax OR capton OR captopren OR captoprilan OR captoril OR captral OR cardiopril OR cardipril OR catona OR catoplin OR catopril OR cesplon OR cryopril OR debax OR dexacap OR dextro OR ecapres OR ecaten OR epicordin OR epsitron OR farcopril OR farmoten OR hiperil OR hypopress OR hypotensOR OR insucar OR iopril OR isopresol OR katopil OR ketanine OR keyerpril OR lapril OR locap OR lopirin OR lopril OR medepres OR midrat OR minitent OR nolectin OR oltens OR petacilon OR praten OR primace OR proline OR ropril OR smarten OR tenofax OR tensicap OR tensiomen OR tensiomin OR tensobon OR tensoprel OR tensORil OR tenzib OR topace OR toprilem OR typril‐ace OR vasosta OR zapto OR zorkaptil):TI,AB AND CENTRAL:TARGET #56 (ceronapril OR novopril):TI,AB AND CENTRAL:TARGET #57 (cilazapril OR cilazipril OR cilazapril OR dynorm OR inhibace OR inibace OR initiss OR inocar OR justor OR vascace OR vascase):TI,AB AND CENTRAL:TARGET #58 (delapril OR adecut OR alindapril OR deacetylalacepril OR derapril):TI,AB AND CENTRAL:TARGET #59 (enalapril OR renitec OR renitek):TI,AB AND CENTRAL:TARGET #60 (enalaprilat OR enalaprilic OR Vasotec OR xanef):TI,AB AND CENTRAL:TARGET #61 (epicaptopril OR fasidotril OR alatriopril):TI,AB AND CENTRAL:TARGET #62 (fosinopril OR acenor‐m OR bpnorm OR dynacil OR eliten OR fosenopril OR fosinil OR fosinonorm OR fosinorm OR fosipres OR fositen OR fositens OR fovas OR fozitec OR hiperlex OR monopril OR newace OR sapril OR staril OR tenso stop OR tensocardil OR vasopril):TI,AB AND CENTRAL:TARGET #63 (foroxymithine OR gemopatrilat OR libenzapril OR idapril OR imidapril OR novarok OR tanapril OR tanatril OR Indolapril OR indalapril):TI,AB AND CENTRAL:TARGET #64 (lisinopril OR acerbon OR alapril OR alfaken OR carace OR cipril OR coric OR dapril OR fibsol OR inopril OR linopril OR linvas OR lipril OR lisi abz OR lisibeta OR lisigamma OR lisihexal OR lisipril OR lisodur OR lisopress OR lisopril OR lisoril OR lispril OR listril OR lysinopril OR noperten OR novatec OR presiten OR prinil OR prinivil OR qbrelis OR sinopril OR tensopril OR tensyn OR vivatec OR zestomax OR zestril):TI,AB AND CENTRAL:TARGET #65 (moexipril OR fampress OR femipres OR fempres OR fempress OR frempress OR moex OR perdix OR primoxil OR tensotec OR univasc OR univase):TI,AB AND CENTRAL:TARGET #66 (moveltipril OR pentopril):TI,AB AND CENTRAL:TARGET #67 (omapatrilat OR vanlev OR pentropril OR pivopril OR pivalopril):TI,AB AND CENTRAL:TARGET #68 (perindopril OR armix arginin OR bioprexanil OR coverex OR coversoral OR coversum OR coversyl OR mariper OR perindoprilarginin OR perineva OR perstarium OR pirindopril OR prestarium OR prexanil OR procaptan):TI,AB AND CENTRAL:TARGET #69 (quinapril OR accupril OR accuprin OR accupro OR accupron OR acequin OR acuitel OR acuprel OR acupril OR asig OR conan OR ectren OR korec OR quinalapril OR quinaten OR quinazil OR quinhexal OR quinipril):TI,AB AND CENTRAL:TARGET #70 (ramipril OR acovil OR altace OR carasel OR cardace OR corpril OR delix OR hypren OR hytren OR lostapres OR pramace OR ramace OR ramilich OR triatec OR tritace OR unipril OR vesdil OR vivace OR zabien):TI,AB AND CENTRAL:TARGET #71 (rentiapril OR fentiapril OR pentiapril OR s‐nitrosocaptopril OR temocapril):TI,AB AND CENTRAL:TARGET #72 (spirapril OR equaten OR quadropil OR quadropril OR renormax OR renpress OR sandopril OR wandopres):TI,AB AND CENTRAL:TARGET #73 (temocapril OR acecol):TI,AB AND CENTRAL:TARGET #74 (teprotide OR bradykinin potentiating OR nonapeptide converting enzyme inhibitor* OR pyroglutamyltryptophylprolylarginylprolylglutaminylisoleucylprolylproline):TI,AB AND CENTRAL:TARGET #75 (trandolapril OR gopten OR mavik OR odace OR odric OR odrik OR udrik OR utibapril OR zabicipril OR zofenopril OR bifril OR zofenil OR zofenoprilum OR zopranol):TI,AB AND CENTRAL:TARGET #76 (#47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75) #77 MESH DESCRIPTOR Angiotensin Receptor Antagonists EXPLODE ALL AND CENTRAL:TARGET #78 (angiotensin NEAR3 receptor antagon*):TI,AB AND CENTRAL:TARGET #79 (angiotensin NEAR3 receptor block*):TI,AB AND CENTRAL:TARGET #80 (arb OR arbs):TI,AB AND CENTRAL:TARGET #81 (sartan OR sartans):TI,AB AND CENTRAL:TARGET #82 (azilsartan OR edarbi OR ipreziv):TI,AB AND CENTRAL:TARGET #83 candesartan:TI,AB AND CENTRAL:TARGET #84 (elisartan OR embusartan OR forasartan OR milfasartan OR pratosartan OR KT3‐671 OR saprisartan OR zolasartan):TI,AB AND CENTRAL:TARGET #85 (eprosartan OR epratenz OR futuran OR naviten OR navixen OR regulaten OR tevesten OR tevetan OR teveten OR tevetenz):TI,AB AND CENTRAL:TARGET #86 (irbesartan OR approvel OR aprovel OR arbez lr OR avapro OR ifirmasta OR Irban OR irbetan OR iretensa OR irovel OR irvell OR karvea):TI,AB AND CENTRAL:TARGET #87 (losartan OR cozaar):TI,AB AND CENTRAL:TARGET #88 (olmesartan OR alteis OR belsar OR benetor OR benevas OR benicar OR ixia OR laresin OR mencord OR mesar OR olartan OR olmeblo OR olmec OR olmes OR olmetec OR olpress OR olsar OR omesar OR openvas OR plaunac OR santini OR sarten OR tensar OR tensiol OR vivactra OR votum):TI,AB AND CENTRAL:TARGET #89 (tasosartan OR anazor OR verdia):TI,AB AND CENTRAL:TARGET #90 (telmisartan OR actelsar OR "kinzal mono" OR kinzalmono OR micardis OR predxal OR pritor OR pritoral OR semintra OR tolura):TI,AB AND CENTRAL:TARGET #91 (valsartan OR angiosan OR cordinate OR dalzad OR diovan OR diovane OR kalpress OR miten OR nisis OR prexxartan OR provas OR rixil OR saval OR tareg OR tazea OR troval OR valpression OR vals OR valsocard OR valtan OR valtsu):TI,AB AND CENTRAL:TARGET #92 (#77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91) #93 MESH DESCRIPTOR Renin WITH QUALIFIER AI AND CENTRAL:TARGET #94 (RAS NEAR2 inhibit*):TI,AB AND CENTRAL:TARGET #95 (renin NEAR2 inhibit*):TI,AB AND CENTRAL:TARGET #96 (aliskiren OR enviage OR rasilez OR riprazo OR sprimeo OR tekturna):TI,AB AND CENTRAL:TARGET #97 ciprokiren:TI,AB AND CENTRAL:TARGET #98 remikiren:TI,AB AND CENTRAL:TARGET #99 terlakiren:TI,AB AND CENTRAL:TARGET #100 zankiren:TI,AB AND CENTRAL:TARGET #101 (#93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100) #102 MESH DESCRIPTOR Calcium Channel Blockers EXPLODE ALL AND CENTRAL:TARGET #103 ((calcium NEAR2 antagonist*) OR (calcium NEAR2 block*) OR (calcium NEAR3 inhibit*)):TI,AB AND CENTRAL:TARGET #104 (amlodipine OR amdip OR amloc OR amlodipin OR amlodipina OR amlodis OR amlopin OR amlor OR astudal OR istin OR levamlodipine OR norvasc):TI,AB AND CENTRAL:TARGET #105 (amrinone OR amcoral OR amrinone OR cardiotone OR cartonic OR cordemcura OR inamrinone OR inocor OR vesistol OR wincoram):TI,AB AND CENTRAL:TARGET #106 (aranidipine OR bec OR sapresta):TI,AB AND CENTRAL:TARGET #107 (bencyclane OR bencyclan OR benzcyclan OR benzyclane OR desoblit OR dilangio compositu OR fludilat OR fluxema OR halidor OR ludilat):TI,AB AND CENTRAL:TARGET #108 (benidipine OR coniel):TI,AB AND CENTRAL:TARGET #109 (bepridil OR angopril OR bedapin OR bepadin OR bepricol OR cordium OR cruor OR unicordium OR vascor):TI,AB AND CENTRAL:TARGET #110 (cilnidipine OR atelec OR cinaldipine OR cinalong OR siscard):TI,AB AND CENTRAL:TARGET #111 (cinnarizine OR aplactan OR aplexal OR apomitere OR apotomin OR artate OR carecin OR cerebolan OR cerepar OR cibine OR cimarizine OR cinabioquim OR cinaperazine OR cinarizina OR cinarizine OR cinaziere OR cinazyn OR cinna OR cinnabene OR cinnacet OR cinnaforte OR cinnageron OR cinnarazine OR cinnarizin OR cinnipirine OR cinniprine OR cisaken OR corathiem OR denapol OR dimitron OR dimitronal OR eglen OR giganten OR glanil OR hilactan OR ixertol OR katoseran OR labyrin OR lazeta OR marisan OR midronal OR mitronal OR olamin OR processine OR roin OR sedatromin OR sepan OR siptazin OR spaderizine OR stugeron OR stutgeron OR stutgin):TI,AB AND CENTRAL:TARGET #112 (clentiazem OR logna OR darodipine OR dazodipine):TI,AB AND CENTRAL:TARGET #113 (diltiazem OR acalix OR adizem OR aldizem OR altiazem OR anginyl OR angiodrox OR angiotrofen OR angiotrofin OR angiozem OR angitil OR angizem OR angoral OR anoheal OR anotrit OR anzem OR auscard OR balcor OR beatizem OR blocalcin OR britiazim OR bruzem OR calcicard OR calnurs OR cardcal OR cardiazem OR cardiben OR cardiem OR cardil OR cardiosta OR cardium OR cardizem OR carex OR carreldon OR cartia xt OR cascor xl OR cirilen OR coras OR cordizem OR dazil OR deltazen OR diacor OR diatal OR diazem OR dilacor OR diladel OR dilatam OR dilatame OR dilcard OR dilcardia OR dilem OR dilfar OR dilgard OR diloc OR dilren OR dilrene OR dilso OR "dilt‐cd" OR diltahexal OR diltam OR diltan OR diltelan OR diltia OR diltiamax OR diltiasyn OR diltime OR diltiwas OR diltzac OR dilzanton OR dilzem OR dilzene OR dilzereal OR dilzicardin OR dinisor OR doclis OR dodexen OR dyalac OR entrydil OR etizem OR filazem OR gadoserin OR grifodilzem OR hagen OR helsibon OR herben OR herbesser OR hesor OR incoril OR kaizem OR kenzem OR lacerol OR levodex OR levozem OR lytelsen OR masdil OR miocardie OR monotildiem OR myonil OR pazeadin OR presoken OR slozem OR surazem OR tazem OR taztia OR tiadil OR tiamate OR tiazac OR tilazem OR tildiem OR tilker OR vasmulax OR vasocardol OR wentizem OR zandil OR zatim OR zemret OR zemtrial OR zildem OR ziruvate):TI,AB AND CENTRAL:TARGET #114 (efonidipine OR finte OR landel OR elgodipine):TI,AB AND CENTRAL:TARGET #115 (etafenone OR baxacor OR dialicor OR diethylaminoethoxyphenylpropiophenone hydrochloride OR etafenon OR etaphenone OR kca OR revisor):TI,AB AND CENTRAL:TARGET #116 fantofarone:TI,AB AND CENTRAL:TARGET #117 (felodipine OR agon OR dilahex OR dilofen OR dilopin OR fedil OR felim OR felo biochemie OR felo er OR felo‐basf OR felo puren OR felobal OR felobeta OR felocor OR felodipin OR felodur OR felogamma OR felogard OR felop OR felopine‐sr OR fensel OR flodil OR h15482 OR hydac OR keydipin OR lodistad OR modip OR munobal OR nirmadil OR penedil OR perfudal OR plendil OR preslow OR prevex OR renedil OR selepine OR splendil OR versant xr):TI,AB AND CENTRAL:TARGET #118 (fendiline OR cordan OR digosensit OR fendilar OR fendiline OR phendiline OR sensit OR senzit):TI,AB AND CENTRAL:TARGET #119 (flunarizine OR flunarizine OR sibelium):TI,AB AND CENTRAL:TARGET #120 (gallopamil OR elgiprona OR galcan OR gallobeta OR gallopamile OR methoxyverapamil OR prebet OR procorum):TI,AB AND CENTRAL:TARGET #121 (isradipine OR dynacirc OR icaz OR isrodipine OR lomir OR prescal OR vascal):TI,AB AND CENTRAL:TARGET #122 (lacidipine OR aponil OR caldine OR lacimen OR lacipil OR lacirex OR lacitens OR motens OR viapres):TI,AB AND CENTRAL:TARGET #123 (lercanidipine OR carmen OR corifeo OR lercadip OR lercan OR lerdip OR masnidipine OR zanedip OR zanidip):TI,AB AND CENTRAL:TARGET #124 (lidoflazine OR clinium OR ordiflazine):TI,AB AND CENTRAL:TARGET #125 (lomerizine OR migsis OR terranas):TI,AB AND CENTRAL:TARGET #126 (manidipine OR artedil OR calslot OR franidipine OR iperten OR manyper OR vascoman):TI,AB AND CENTRAL:TARGET #127 (mepirodipine OR barnidipine OR cyress OR hypoca OR libradin OR vasexten):TI,AB AND CENTRAL:TARGET #128 (mibefradil OR posicor):TI,AB AND CENTRAL:TARGET #129 (nicardipine OR antagonil OR barizine OR bionicard OR cardene OR cardepine OR cardibloc OR cardipene OR dacarel OR dagan OR flusemide OR karden OR lecibral OR lincil OR loxen OR lucenfal OR nerdipine OR nicardal OR nicardil OR nicardipino OR nicarpin OR nicodel OR nimicor OR perdipina OR perdipine OR ranvil OR ridene OR roxen OR rycarden OR rydene OR saf card OR vasodin OR vasonase):TI,AB AND CENTRAL:TARGET #130 (nifedipine OR adalat OR cordipin OR cordipine OR corinfar OR fenigidin OR korinfar OR nifangin OR procardia OR vascard):TI,AB AND CENTRAL:TARGET #131 niguldipine:TI,AB AND CENTRAL:TARGET #132 (nilvadipine OR escor OR nilvadil OR nivadil OR nivadipine):TI,AB AND CENTRAL:TARGET #133 (nimodipine OR admon OR brainal OR calnit OR eugerial OR grifonimod OR kenesil OR kenzolol OR modus OR nidip OR nimodilat OR nimodipin OR nimodipin‐isis OR nimodipino OR nimotop OR nisom OR nymalize OR periplum OR remontal OR tropocer OR vasoflex OR vasotop):TI,AB AND CENTRAL:TARGET #134 (nisoldipine OR angiolat OR baymycard OR corasol OR nisoldin OR sular OR syscor):TI,AB AND CENTRAL:TARGET #135 (nitrendipine OR balminil OR baylotensin OR bayotensin OR baypresol OR baypress OR gericin OR jutapress OR lusopress OR nidrel OR niprina OR nitre OR nitregamma OR nitren OR nitrend ksk OR nitrepress OR nitre‐puren OR nitrepuren OR nitrendepat OR nitrendi biochemie OR nitrendidoc OR nitrendimerck OR nitrendipin OR nitrendipin‐corax OR nitrendipin‐ratiopharm OR nitrendipincorax OR nitrendipino OR nitrendipinratiopharm OR nitrensal OR tensogradal OR trendinol OR vastensium):TI,AB AND CENTRAL:TARGET #136 (perhexilene OR perhexiline):TI,AB AND CENTRAL:TARGET #137 (prenylamine OR corontin OR difril OR segontin):TI,AB AND CENTRAL:TARGET #138 (semotiadil OR levosemotiadil OR sesamodil):TI,AB AND CENTRAL:TARGET #139 (terodiline OR biro OR mictrol OR micturin):TI,AB AND CENTRAL:TARGET #140 (tiapamil OR dimeditiapramine OR larocORd OR thiapamil OR verocainide OR verocainine):TI,AB AND CENTRAL:TARGET #141 (verapamil OR apo‐verap OR apoacor OR arpamyl OR azupamil OR berkatens OR calan OR calaptin OR cardiagutt OR cardibeltin OR cardiolen OR cardiovert OR caveril OR cintsu OR civicor OR coraver OR cordilat OR cordilox OR corpamil OR covera OR dexverapamil OR dignover OR dilacoran OR dilacoron OR durasoptin OR falicard OR finoptin OR flamon OR geangin OR hexasoptin OR ikacor OR ikakor OR ikapress OR iproveratril OR iso‐card sr OR isoptin OR isoptine OR isoptino OR izoptin OR lekoptin OR manidon OR napamil OR novapamyl OR novo veramil OR novopressan OR phynoptin OR quasar OR ravamil OR securon OR univer OR vasolan OR vasomil OR vasopten OR verabeta OR veracaps sr OR veracor OR verahexal OR veraloc OR veramex OR veramil OR verapin OR verapress OR veratad OR verdilac OR verelan OR verexamil OR veroptin stada OR verpamil OR vetrimil OR vortac OR zolvera):TI,AB AND CENTRAL:TARGET #142 (#102 OR #103 OR #104 OR #105 OR #106 OR #107 OR #108 OR #109 OR #110 OR #111 OR #112 OR #113 OR #114 OR #115 OR #116 OR #117 OR #118 OR #119 OR #120 OR #121 OR #122 OR #123 OR #124 OR #125 OR #126 OR #127 OR #128 OR #129 OR #130 OR #131 OR #132 OR #133 OR #134 OR #135 OR #136 OR #137 OR #138 OR #139 OR #140 OR #141) #143 MESH DESCRIPTOR Adrenergic Beta‐Antagonists EXPLODE ALL AND CENTRAL:TARGET #144 ((beta NEAR2 adrenergic*) OR (beta NEAR2 antagonist*)):TI,AB AND CENTRAL:TARGET #145 ((beta NEAR2 block*) OR (beta NEAR2 receptor*)):TI,AB AND CENTRAL:TARGET #146 (acebutolol OR acecor OR acetobutolol OR apoacebutolol OR diasectral OR espesil OR flebutol OR grifobutol OR monitan OR neptal OR neptall OR novoacebutolol OR prent OR rhotral OR sectral):TI,AB AND CENTRAL:TARGET #147 (alprenolol OR alfeprol OR alloprenalol OR alpheprol OR alprendol OR alprenol OR alprenololum OR apliobal OR aprenolol OR aptia OR aptin OR aptin‐duriles OR aptinduriles OR aptina OR aptine OR aptol OR astra OR betacard OR betapin):TI,AB AND CENTRAL:TARGET #148 (amosulalol OR amsolulol OR howgan OR lowgan):TI,AB AND CENTRAL:TARGET #149 (arotinolol OR almarl):TI,AB AND CENTRAL:TARGET #150 (atenolol OR ablok OR adoll OR alonet OR altol OR anolene OR anolpin OR anselol OR arandin OR asten OR atarox OR atcardil OR atecard OR atehexal OR atelol OR atenblock OR atendol OR atenet OR ateni OR atenil OR ateno OR atenogamma OR atenol OR atereal OR aterol OR atestad OR atinol OR atolmin OR b‐vasc OR betablok OR betacar OR betarol OR betatop ge OR beten OR bloket OR blokium OR blotex OR cardioten OR catenol OR coratol OR corotenol OR durabeta OR esatenolol OR evitocor OR farnormin OR felo‐bits OR hypernol OR hypoten OR internolol OR lo‐ten OR loten OR lotenal OR martenol OR mirobect OR myocord OR neotenol OR nolol OR normalol OR normaten OR normiten OR nortelol OR noten OR oraday OR ormidol OR paesumex OR plenacor OR preloc OR premorine OR prenolol OR prenormine OR ranlol OR rozamin OR serten OR stermin OR temoret OR tenblok OR tenidon OR tenoblock OR tenocor OR tenol OR tenolin OR tenolol OR tenopress OR tenoprin OR tenormin OR tenormine OR tenostat OR tensig OR tensinor OR ternolol OR therabloc OR tredol OR vascoten OR velorin OR vericordin OR wesipin):TI,AB AND CENTRAL:TARGET #151 (befunolol OR befnolol OR benfuran OR bentos OR bentox OR glauconex OR glaukonex):TI,AB AND CENTRAL:TARGET #152 (betaxolol OR "alcon betoptic" OR betac OR betarun OR betasel OR betaxon OR betoptic OR betoptima OR betoquin OR kerlon OR kerlone OR kerlong OR levobetaxolol OR lokren OR optibet OR optipress OR oxodal OR tonobexol):TI,AB AND CENTRAL:TARGET #153 (bevantolol OR benatol OR bevantololum OR calvan OR ranestol OR vantol):TI,AB AND CENTRAL:TARGET #154 (bisoprolol OR concor):TI,AB AND CENTRAL:TARGET #155 (bopindolol OR sandonorm OR wandonorm):TI,AB AND CENTRAL:TARGET #156 bucindolol:TI,AB AND CENTRAL:TARGET #157 (bucumolol OR bucumarol OR bucumolol):TI,AB AND CENTRAL:TARGET #158 (bufarolol OR angium):TI,AB AND CENTRAL:TARGET #159 (bufetolol OR adbiol):TI,AB AND CENTRAL:TARGET #160 (bunitrolol OR betrilol OR stresson OR tilcant):TI,AB AND CENTRAL:TARGET #161 butofilolol:TI,AB AND CENTRAL:TARGET #162 (carazolol OR conducton OR suacron):TI,AB AND CENTRAL:TARGET #163 (carteolol OR arteolol OR arteoptic OR arteoptik OR caltamol OR calte OR carbonolol OR carteabak OR carteol OR cartrol OR elebloc OR endak OR glauteolol OR karol OR karteol OR mikelan OR ocupress OR stobol OR teoptic):TI,AB AND CENTRAL:TARGET #164 (carvedilol OR cardiol OR cardivas OR carvedlol OR carvipress OR carvrol OR coreg OR dilatrend OR dilbloc OR dimitone OR eucardic OR kredex OR quarto OR quarto OR v‐bloc):TI,AB AND CENTRAL:TARGET #165 (celiprolol OR abecor OR celectol OR dilanorm OR selecor OR selectol):TI,AB AND CENTRAL:TARGET #166 (cetamolol OR betacor):TI,AB AND CENTRAL:TARGET #167 (cloranolol OR tobanum):TI,AB AND CENTRAL:TARGET #168 (epanolol OR visacor):TI,AB AND CENTRAL:TARGET #169 (esmolol OR brevibloc OR miniblock):TI,AB AND CENTRAL:TARGET #170 (indenolol OR pulsan):TI,AB AND CENTRAL:TARGET #171 (labetalol OR abetol OR albetol OR amipress OR apolabetalol OR biascor OR dilevalol OR dilevalon OR hybloc OR ibidomide OR ipolab OR labelol OR labesine OR lamitol OR levadil OR liondox OR normodyne OR presdate OR presolol OR pressalolo OR salmagne OR trandate OR unicard):TI,AB AND CENTRAL:TARGET #172 (levobunolol OR "ak beta" OR akbeta OR apolevobunolol OR betagan OR betasite OR bunolgan OR gotensin OR l bunolol hydrochloride OR levo bunolol OR novolevobunolol OR pmslevobunolol OR ultracortenol OR vistagan OR vistagen):TI,AB AND CENTRAL:TARGET #173 (mepindolol OR betagon OR caridian OR corindolan):TI,AB AND CENTRAL:TARGET #174 (metipranolol OR beta ophtiole OR betalol OR betamann OR betamet OR betanol OR betanolol OR disorat OR glauline OR methypranol OR normoglaucon mite OR ophtiole OR optipranolol OR trimepranol):TI,AB AND CENTRAL:TARGET #175 (metoprolol OR beloc duriles OR belok zok OR betaloc OR betalok OR lopressor OR seloken OR spesicor OR spesikor OR toprol):TI,AB AND CENTRAL:TARGET #176 moprolol:TI,AB AND CENTRAL:TARGET #177 (nadolol OR betadol OR corgard OR farmagard OR nadic OR solgol OR solgol):TI,AB AND CENTRAL:TARGET #178 nadoxolol:TI,AB AND CENTRAL:TARGET #179 (nebivolol OR bivolet OR bystolic OR dexnebivolol OR levonebivolol OR lobivon OR narbivolol OR nebilet OR nebilox OR silostar):TI,AB AND CENTRAL:TARGET #180 (nifenalol OR "d inpea" OR "dextro inpea" OR dextro levo inpea OR inpea OR isophenethanol OR isophenethyl alcohol OR isopropylaminonitrophenylethanol OR nefenalol):TI,AB AND CENTRAL:TARGET #181 (oxprenolol OR cordexol OR coretal OR koretal OR oxtrenolol OR tevacor OR trasacor OR trasicor):TI,AB AND CENTRAL:TARGET #182 pafenolol:TI,AB AND CENTRAL:TARGET #183 (penbutolol OR betapresin OR betapressin OR blocotin OR levatolor paginol):TI,AB AND CENTRAL:TARGET #184 (pindolol OR barbloc OR betapindol OR blocklin OR calvisken OR carvisken OR decreten OR dranolis OR durapindol OR hexapindol OR hydroxypropylaminopropoxyindol OR nonspi OR novo‐pindol OR pectobloc OR pectoblock mite OR pidol OR pinbetol OR pinden OR pindol OR pindomex OR pindoreal OR pinloc OR pinolol OR pinsken OR prindolol OR prinodolol OR pyndale OR treparasen OR viskeen OR visken OR viskene OR vypen):TI,AB AND CENTRAL:TARGET #185 (practolol OR dalzic OR eraldin OR practolole OR praktolol OR praktololu OR proctalol OR teranol):TI,AB AND CENTRAL:TARGET #186 (pronethalol OR alderlin OR nethalide OR pronetal OR pronetalol):TI,AB AND CENTRAL:TARGET #187 (propranolol OR acifol OR adrexan OR alperol OR anaprilin OR anapriline OR anaprilinium OR anapryline OR angilol OR apsolol OR arcablock OR artensol OR authus OR avlocardyl OR becardin OR bedranol OR beprane OR bercolol OR berkolol OR beta neg OR beta tablinen OR beta timelets OR betabloc OR betadipresan OR betadren OR betaneg OR betaprol OR betares OR betaryl OR blocard OR blocaryl OR cardinol OR ciplar OR corbeta OR deralin OR dexpropranolol OR dibudinate OR dideral OR dociton OR docitone OR durabeton OR duranol OR efektolol OR elbrol OR emforal OR farmadral OR farprolol OR frekven OR frina OR hemangeol OR hemangiol OR hopranolol OR ikopal OR Impral OR Inderal OR inderalici OR inderex OR indicardin OR indobloc OR innopran OR inpanol OR ipran OR lederpronol OR levopropranolol OR napriline OR noloten OR obsidan OR obsin OR obzidan OR oposim OR phanerol OR prandol OR prano puren OR pranopuren OR prestORal OR prolol OR pronovan OR propabloc OR propal OR propalong OR propayerst OR propercuten OR prophylux OR propra ratiopharm OR propral OR propranur OR proprasylyt OR proprasylyte OR reducor OR rexigen OR sagittol OR stapranolol OR sumial OR tenomal OR tensiflex OR waucoton):TI,AB AND CENTRAL:TARGET #188 (sotalol OR alosot OR beta‐cardone OR betacardone OR betades OR betapace OR bonpro OR corsotalol OR darob OR dexsotalol OR dextrosotalol OR favorex OR gilucor OR hipecor OR isotalol OR jutalex OR levosotalol OR rentibloc OR rotalol OR satalol OR satolol OR solavert OR sorine OR sota saar OR sotab OR sotabeta OR sotacol OR sotacor OR sotahexal OR sotalex OR sotaper OR sotapor OR sotastad OR sotylize OR tachytalol):TI,AB AND CENTRAL:TARGET #189 (sulfinalol OR talinolol OR cordanum):TI,AB AND CENTRAL:TARGET #190 (tertatolol OR artex OR prenalex):TI,AB AND CENTRAL:TARGET #191 (tilisolol OR daim OR selecal):TI,AB AND CENTRAL:TARGET #192 (timolol OR apotimolol OR blocadren OR optimal OR timacar OR timolo OR timoptic OR timoptol OR titol):TI,AB AND CENTRAL:TARGET #193 (toliprolol OR doberol):TI,AB AND CENTRAL:TARGET #194 xibenolol:TI,AB AND CENTRAL:TARGET #195 (#143 OR #144 OR #145 OR #146 OR #147 OR #148 OR #149 OR #150 OR #151 OR #152 OR #153 OR #154 OR #155 OR #156 OR #157 OR #158 OR #159 OR #160 OR #161 OR #162 OR #163 OR #164 OR #165 OR #166 OR #167 OR #168 OR #169 OR #170 OR #171 OR #172 OR #173 OR #174 OR #175 OR #176 OR #177 OR #178 OR #179 OR #180 OR #181 OR #182 OR #183 OR #184 OR #185 OR #186 OR #187 OR #188 OR #189 OR #190 OR #191 OR #192 OR #193 OR #194) #196 MESH DESCRIPTOR Adrenergic alpha‐Antagonists EXPLODE ALL AND CENTRAL:TARGET #197 ((adrenergic NEAR2 alpha) OR (adrenergic NEAR2 antagonist*)):TI,AB AND CENTRAL:TARGET #198 ((adrenergic NEAR2 block*) OR (alpha NEAR2 block*) OR (receptor* NEAR2 block*)):TI,AB AND CENTRAL:TARGET #199 (alpha NEAR2 receptor antagonist*):TI,AB AND CENTRAL:TARGET #200 (alfuzosin OR bunazosin OR metazosin OR neldazosin OR silodosin OR terazosin OR tiodazosin OR trimazosin):TI,AB AND CENTRAL:TARGET #201 (doxazosin* OR alfamedin OR cardular OR cardura OR carduran OR diblocin OR doxa puren OR doxauro OR doxacor OR doxagamma OR doxamax OR doxatensa OR doxazomerck OR doxazosina OR jutalar OR progandol OR uriduct OR uroprost OR zoxan):TI,AB AND CENTRAL:TARGET #202 (prazosin OR adversuten OR alpress OR atodel OR decliten OR deprazolin OR duramipress OR eurex OR furazosin OR hexapress OR hypotens OR hypovase OR hyprosin OR lentopres OR minipres OR minesin OR minipres OR minipress OR minison OR mizosin OR patsolin OR peripress OR prasig OR pratisol OR pratsiol OR prazac OR prazopress OR prazosine OR pressin OR prozosin):TI,AB AND CENTRAL:TARGET #203 (tamsulosin OR alna OR amsulosin OR flomax OR flomaxtra OR hamal OR josir OR mapelor OR mecir OR omexel OR omic OR omix OR omnic OR pradif OR secotex OR tamfrex OR urolosin):TI,AB AND CENTRAL:TARGET #204 (#196 OR #197 OR #198 OR #199 OR #200 OR #201 OR #202 OR #203) #205 ((#46 AND #76) OR (#46 AND #92) OR (#46 AND #101) OR (#46 AND #142) OR (#46 AND #195) OR (#46 AND #204) OR (#76 AND #92) OR (#76 AND #101) OR (#76 AND #142) OR (#76 AND #195) OR (#76 AND #204) OR (#92 AND #101) OR (#92 AND #142) OR (#92 AND #195) OR (#92 AND #204) OR (#101 AND #142) OR (#101 AND #195) OR (#101 AND #204) OR (#142 AND #195) OR (#142 AND #204) OR (#195 AND #204)) #206 MESH DESCRIPTOR Drug Therapy, Combination AND CENTRAL:TARGET #207 (add* OR combin* OR multipl* OR plus OR polytherap* OR versus OR vs):TI,AB AND CENTRAL:TARGET #208 (#206 OR #207) #209 MESH DESCRIPTOR Hypertension AND CENTRAL:TARGET #210 MESH DESCRIPTOR Essential Hypertension AND CENTRAL:TARGET #211 hypertens*:TI,AB AND CENTRAL:TARGET #212 ((elevat* OR high OR rais*) NEAR2 (arterial pressur* OR blood pressur* OR diastolic pressur* OR systolic pressur*)):TI,AB AND CENTRAL:TARGET #213 ((elevat* OR high OR rais*) NEAR2 (bp OR dbp OR sbp)):TI,AB AND CENTRAL:TARGET #214 (#209 OR #210 OR #211 OR #212 OR #213) #215 #205 AND #208 AND #214 #216 (MESH DESCRIPTOR Hypertension, Pregnancy‐Induced EXPLODE ALL OR MESH DESCRIPTOR Hypertension, Pulmonary OR MESH DESCRIPTOR Hypertension, Portal OR MESH DESCRIPTOR Hypertension, Renovascular OR MESH DESCRIPTOR Obstetrics EXPLODE ALL OR MESH DESCRIPTOR Ocular Hypertension EXPLODE ALL OR MESH DESCRIPTOR Pregnancy EXPLODE ALL OR MESH DESCRIPTOR Pregnancy Complications EXPLODE ALL OR MESH DESCRIPTOR Prenatal Education EXPLODE ALL) AND CENTRAL:TARGET #217 (anaesth* OR anesth* OR ante natal* OR antenatal* OR bleeding OR caesarean OR cesarean OR caesarian OR cesarian OR caesarien OR cesarien OR cirrho* OR eclamp* OR epidural OR fetal OR foetal OR fetus OR foetus OR gestational hypertens* OR glaucom* OR interocular OR intraocular OR intracran* OR intuba* OR lactation* OR neonat* OR new born* OR newborn* OR obstetr* OR ocular OR ophthalm* OR peri partum OR peripartum OR portal hypertens* OR portopulmonary OR pre eclamp* OR preeclamp* OR pregnancies OR pregnancy OR pregnant OR pre natal* OR prenatal* OR pulmonary hypertens* OR pulmonary arterial hypertens* OR secondary hypertens* OR spinal):TI AND CENTRAL:TARGET #218 #215 NOT (#216 OR #217)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2021 March 10> Search Date: 11 March 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp *thiazide diuretic agent/
2 exp *loop diuretic agent/
3 thiazide*.tw.
4 ((sodium chloride adj2 cotransporter inhibit*) or (sodium chloride adj2 co‐transporter inhibit*) or (sodium chloride adj2 symporter inhibit*)).tw.
5 ((ceiling adj2 diuretic*) or (loop adj2 diuretic*) or (potassium‐depleting adj2 diuretic*)).tw.
6 amiloride/ or (amiloride or amiclaran or amidal or amiduret trom or amikal or amiloberag or amilorid or amiloridehydrochlorhydrate or amiloridine or amipramidine or amyloride or arumil or berkamil or colectril or guanamprazine or kaluril or medamor or midamor or midoride or modamide or nirulid or pandiuren).tw.
7 azosemide/ or (azosemide or azosemid or luret).tw.
8 exp benzothiadiazine derivative/ or benzothiadiazine*.tw.
9 bendroflumethiazide/ or (bendroflumethiazide or aprinox or bendrofluazide or bendroflumethiazide or benzhydroflumethiazide or benzydroflumethiazide or benzyl hydroflumethiazide or benzylhydroflumethiazide or benzide or berkozide or bristuron or centonuron or centyl or esberizid or naturetin or naturine or neo naclex or neonaclex or naturetin or naturine or neonadex or pluryl or pluryle or repicin or salures or sinesalin or urizid).tw.
10 bumetanide/ or (bumetanide or budema or bumedyl or bumelex or bumet or bumetamide or bumethanide or bumetidine or bumex or burinax or burinex or busix or butinat or butinon or bymex or cambiex or drenural or farmadiuril or fontego or fordiuran or lixil or lunetoron or miccil or primex).tw.
11 butizide/ or (butizide or buthiazide or eunephran or eunepran or isobutylhydrochlorothiazide or modenol or saltucin or thiabulazid or thiabutazide or thiobulazid or tiabutazide).tw.
12 chlorothiazide/ or (chlorothiazide or chlorosal or chlorothiazid or chlorothiazidum or chlorothiazine or chlorthiazide or chlotride or diachlor or diuril or diurilix or diuriwas wassermann milano or flumen or lyovac or saluric or warduzuide).tw.
13 chlorthalidone/ or (chlorthalidone or aquadon or chlorphthalidolone or chlortalidon or chlortalidone or clortalidone or chlorthalidine or chlorthalidon or chlorthialidone or clortalil or edemdal or hidronal or higroton or higrotona or hygroton or hylidone or hypertol or hythalton or igrolina or igroton or isoren or natriuran or oxodolin or oxodoline or phthalamidine or phthalamodine or phthalamudine or renon or servidone or thalitone or urandil or urofinil or zambesil).tw.
14 cicletanine/ or (cicletanine or cicletanide or cycletanide or justar or tenstaten or tenstatin).tw.
15 clopamide/ or (clopamide or adurix or aquez or brinaldix or brinaldrix or brinedine or chlosudimeprimylum clopamid or clopamidum or clopamine).tw.
16 clorexolone/ or (clorexolone or anhydron or clorexone or chlorexolone or cyclothizaide or doburil or flonatril or fluidil or klorex or nefrolan or valmiran).tw.
17 cyclopenthiazide/ or (cyclopenthiazide or cyclomethiazide or cyclopenthiazine or cyclopentiazide or navidex or navidrex or navidrix or salimid or tsiklometiazid).tw.
18 ethacrynic acid/ or (ethacrynic acid or edecril or edecrin or edecrina or endecril or etacrinic acid or etacrynate or etacrynic acid or ethacrinic acid or ethacrynate or ethacryonic acid or ethocrynic acid or ethycrynic acid or hydromedin or lyovac sodium edecrin or reomax or sodium ethacrynate or uregit or uregyt).tw.
19 eplerenone/ or (eplerenone or elecor or eplerenon or epoxymexrenone or inspra).tw.
20 etozolin/ or (etozolin or elkapin or etazolin or etozoline or ozolinone ethyl ester).tw.
21 fenquizone/ or (fenquizone or idrolone).tw.
22 furosemide/ or (furosemide or aldic or aluzine or anfuramaide or aquarid or arasemide or cetasix or desal or diamazon or dirine or discoid or diumide or diural or diuresal or diurin or diurix or diurolasa or diusemide or diuspec or dryptal or durafurid or edenol or errolon or eutensin or eutensine or flurosemide or franyl or fretic or frumid or frusedan or frusehexal or frusema or frusemidor frusemide or frusid or fruzex or fumarenid or fumide or furanthril or furantral or furantril or furanturil or furasemide or furesin or furesis or furetic or furix or furmid or furo puren or furo‐basan or furo‐puren or furobasan or furomen or furomex or furomide or furomin or furopuren or furorese or furosamide or furoscan or furose or furosemid or furosemix or furosimide or furosix or furovite or fursemide or fusid or fusimex or hissuflux or hydro rapid or impugan or jufurix or kofuzon or kutrix or lasiletten or lasilix or lasix or laxis or laxur or luramide or marsemide or mirfat or odemase or odemex or oedemase or oedemex or pharmix or promedes or radisemide or rasitol or retep or salinex or seguril or selectofur or sigasalur or uremide or uresix or urex‐m or vesix or zafurida).tw.
23 hydrochlorothiazide/ or (hydrochlorothiazide or apo‐hydro or aquarius or aquazide or bisalunil or bpzide or bremil or chlorosulthiadil or chlorsulfonamidodihydrobenzothiadiazine or cidrex or clothia or dehydratin or diaqua or dichlorosal or dichlothiazide or dichlotride or dichlozid or diclotride or didralin or dihydrochlorothiazide or dihydrodiuril or direma or disaluril or disothiazide or dithiazide or diu melusin or diumelusin or diurace or diurex or esidrex or esidrix or fluvin or hctz or hidrenox or hidril or hidroronol or hidrosaluretil or hudorex or hychlozide or hydrex‐semi or hydril or hydro aquil or hydrochlor or hydrochloro thiazide or hydrochlorothiamide or hydrochlorothiazid or hydrochlorothiazine or hydrochlorzide or hydrochlothiazide or hydro diuril or hydrodiuril or hydromal or hydrororonol or hydro saluric or hydrosaluric or hydrothide or hydro tonuron or hydrozide or hypothiazid or hypothiazide or ivaugan or maschitt or microzide or mictrin or nefrix or neoflumen or newtolide or niagar or oretic or pantemon or ridaq or sectrazide or tandiur or thiadril or thiaretic or thiuretic or urodiazin or urodiazine or urozide or vetidrex).tw.
24 hydroflumethiazide/ or (hydroflumethiazide or bristab or di ademil or diademil or dihydroflumethiazide or diraudixin or diucardin or hiserpin or hydrenox or leodrin or leodrine or metflorylthiadiazine or naclex or rontyl or saluron or sisuril or trifluoromethylhydrothiazide).tw.
25 indapamide/ or (indapamide or agelan or apadex or arifon or damide or dapamax or diflerix or dixamid or extur or fludex or fluidema or frumeron or indahexal or indalix or indamol or indapam or indapress or indicontin or indoline or indopamide or inpamide or insig or ipamix or lorvas or loxide or lozol or metindamide or millibar or naplin or natrilix or natrix or noranat or pamid or pressural or pretanix or rinalix or sicco or tandix or tertensif or veroxil).tw.
26 indacrinone/ or (indacrinone or indacrinic acid or indacrynic acid).tw.
27 mefruside/ or (mefruside or bay caron or baycaron or baycarone or mefrusid).tw.
28 metolazone/ or (metolazone or barolyn or diulo or metalazone or metenix or metolazon or miclox or microx or mykrox or normelan or xuret or zaroxolyn).tw.
29 methyclothiazide/ or (methylclothiazide or aquatensen or enduron or enduron‐m or enduronum or methyclothiazide or methylchlorothiazide or thiazidil).tw.
30 muzolimine/ or (muzolimine or edrul or musolimino).tw.
31 ozolinone.mp.
32 phenoxybenzoic acid.mp.
33 piretanide/ or (piretanide or arelix or arlix or eurelix or lafax or perbilen or tauliz).tw.
34 polythiazide/ or (polythiazide or drenusil or nephril or polythiazide or renese).tw.
35 quinethazone/ or (quinethazone or aquamox or chinethazon or chinethazone or guinethazone or hydromox or kinetazone or quinethazon).tw.
36 spironolactone/ or (spironolactone or abbolactone or acelat or adultmin or alaton or alatone or aldace or aldactone or aldopur or aldospirone or almatol or aquareduct or berlactone or diram or duraspiron or dyta urese or dytaurese or espironolactona or flumach or frumikal or hypazon or idrolattone or jenaspiron or merabis or novospiroton or osiren or osyrol or pirolacton or pondactone or practon or resacton or spiractin or spiridon or spirix or spiro or spiroctan or spirobeta or spirogamma or spirolacton or spirolactone or spirolang or spiron or spirone or spironex or spirono or spironol or spironone or spirospare or spirothiobarbiturate or spirotone or supra puren or suprapuren or uractone or veroshpiron or verospiron or verospirone or xenalon or youlactone).tw.
37 tiamizide/ or (tiamizide or diapamide or thiamizide).tw.
38 ticrynafen/ or (ticrynafen or diflurex or selacryn or selacryn or thienilic acid or thienylic acid or tienilic acid).tw.
39 tizolemide.mp.
40 torasemide/ or (torasemide or demadex or dilutol or diuremid or isemid or isodiur or luprac or presaril or sutril or toradiur or torem or torrem or torsemide or unat or upcard).tw.
41 triamterene/ or (triamterene or dyrenium or dytac or urocaudal or ademin or ademine or dyren or dyrenium or dytac or iatropur or jatropur or noridyl or pterofen or pterophene or teriam or triampterene or triamterence or triamterens or triamteril or triteren or uretren or urocaudal).tw.
42 trichloromethiazide/ or (trichloromethiazide or aquazide or dichloromethylhydrochlorothiazide or diurese or esmarin or eurinol or fluitran or flutra or gangesol or hydrotrichlorothiazide or metahydrin or methahydrin or naqua or naquasone or salurin or triazide or trichlordiuride or trichlorex or trichlormethazide or trichlormethiazide or trichlormas or trichloromethylhydrochlorothiazide or triflumen or wadel).tw.
43 tripamide/ or (tripamide or normonal).tw.
44 xipamide/ or (xipamide or aquaforil or aquaphor or aquaphoril or aquavor or diurexan or lumitens or xipamid or xypamide or zipix).tw.
45 or/1‐44
46 exp *dipeptidyl carboxypeptidase inhibitor/
47 ((angiotensin converting enzyme adj2 antagonist*) or (angiotensin I‐converting enzyme adj2 antagonist*)).tw.
48 ((angiotensin converting enzyme adj2 inhibit*) or (angiotensin I‐converting enzyme adj2 inhibit*)).tw.
49 (ace adj2 inhibit*).tw.
50 (acei or aceis).tw.
51 alacepril/ or (alacepril or alazapril or cetapril).tw.
52 altiopril/ or altiopril calcium/ or (altiopril or lowpress).tw.
53 benazepril/ or (benazepril or benace or boncordin or briem or brien or cibace or cibacen or cibacene or fortekor or lotensin or tenkuoren or zinadril).tw.
54 captopril/ or (captopril or ace‐bloc or acenorm or acepress or acepril or aceprilex or aceril or aceten or adocor or alopresin or altran or apuzin or asisten or capace or capocard or caposan or capoten or capotena or capotril or capril or captace or captensin or capti or captoflux or captohexal or captolane or captomax or capton or captopren or captoprilan or captoril or captral or cardiopril or cardipril or catona or catoplin or catopril or cesplon or cryopril or debax or dexacap or dextro or ecapres or ecaten or epicordin or epsitron or farcopril or farmoten or hiperil or hypopress or hypotensor or insucar or iopril or isopresol or katopil or ketanine or keyerpril or lapril or locap or lopirin or lopril or medepres or midrat or minitent or nolectin or oltens or petacilon or praten or primace or proline or ropril or smarten or tenofax or tensicap or tensiomen or tensiomin or tensobon or tensoprel or tensoril or tenzib or topace or toprilem or typril‐ace or vasosta or zapto or zorkaptil).tw.
55 ceronapril/ or (ceronapril or novopril).tw.
56 cilazapril/ or (cilazapril or cilazipril or cilazapril or dynorm or inhibace or inibace or initiss or inocar or justor or vascace or vascase).tw.
57 delapril/ or (delapril or adecut or alindapril or deacetylalacepril or derapril).tw.
58 enalapril/ or (enalapril or renitec or renitek).tw.
59 enalaprilat/ or (enalaprilat or enalaprilic or Vasotec or xanef).tw.
60 epicaptopril.mp.
61 fasidotril/ or (fasidotril or alatriopril).tw.
62 fosinopril/ or (fosinopril or acenor‐m or bpnorm or dynacil or eliten or fosenopril or fosinil or fosinonorm or fosinorm or fosipres or fositen or fositens or fovas or fozitec or hiperlex or monopril or newace or sapril or staril or tenso stop or tensocardil or vasopril).tw.
63 (foroxymithine or gemopatrilat or libenzapril or idapril).mp.
64 imidapril/ or (imidapril or novarok or tanapril or tanatril).tw.
65 indolapril/ or (indolapril or indalapril).tw.
66 lisinopril/ or (lisinopril or acerbon or alapril or alfaken or carace or cipril or coric or dapril or fibsol or inopril or linopril or linvas or lipril or "lisi abz" or lisibeta or lisigamma or lisihexal or lisipril or lisodur or lisopress or lisopril or lisoril or lispril or listril or lysinopril or noperten or novatec or presiten or prinil or prinivil or qbrelis or sinopril or tensopril or tensyn or vivatec or zestomax or zestril).tw.
67 moexipril/ or (moexipril or fampress or femipres or fempres or fempress or frempress or moex or perdix or primoxil or tensotec or univasc or univase).tw.
68 (moveltipril or pentopril).mp.
69 omapatrilat/ or (omapatrilat or vanlev or pentopril).tw.
70 perindopril/ or (perindopril or armix arginin or bioprexanil or coverex or coversoral or coversum or coversyl or mariper or perindoprilarginin or perineva or perstarium or pirindopril or prestarium or prexanil or procaptan).tw.
71 pivopril/ or (pivopril or pivalopril).tw.
72 quinapril/ or (quinapril or accupril or accuprin or accupro or accupron or acequin or acuitel or acuprel or acupril or asig or conan or ectren or korec or quinalapril or quinaten or quinazil or quinhexal or quinipril).tw.
73 ramipril/ or (ramipril or acovil or altace or carasel or cardace or corpril or delix or hypren or hytren or lostapres or pramace or ramace or ramilich or triatec or tritace or unipril or vesdil or vivace or zabien).tw.
74 rentiapril/ or (rentiapril or fentiapril or pentiapril).tw.
75 s‐nitrosocaptopril.mp.
76 spirapril/ or (spirapril or equaten or quadropil or quadropril or renormax or renpress or sandopril or wandopres).tw.
77 temocapril/ or (temocapril or acecol).tw.
78 teprotide/ or (teprotide or bradykinin potentiating or nonapeptide converting enzyme inhibitor* or pyroglutamyltryptophylprolylarginylprolylglutaminylisoleucylprolylproline).tw.
79 trandolapril/ or (trandolapril or gopten or mavin or odace or odric or odrik or udrik).tw.
80 (utibapril or zabicipril).mp.
81 zofenopril/ or (zofenopril or bifril or zofenil or zofenoprilum or zopranol).tw.
82 or/46‐81
83 exp *angiotensin receptor antagonist/
84 (angiotensin adj3 receptor antagon*).tw.
85 (angiotensin adj3 receptor block*).tw.
86 (arb or arbs).tw.
87 (sartan or sartans).tw.
88 azilsartan/ or azilsartan medoxomil/ or (azilsartan or edarbi or ipreziv).tw.
89 candesartan/ or candesartan.tw.
90 elisartan.mp.
91 embusartan.mp.
92 eprosartan/ or (eprosartan or epratenz or futuran or naviten or navixen or regulaten or tevesten or tevetan or teveten or tevetenz).tw.
93 forasartan.mp.
94 irbesartan/ or (irbesartan or approvel or aprovel or arbez lr or avapro or ifirmasta or Irban or irbetan or iretensa or irovel or irvell or karvea).tw.
95 losartan/ or (losartan or cozaar).tw.
96 milfasartan.mp.
97 olmesartan/ or (olmesartan or alteis or belsar or benetor or benevas or benicar or ixia or laresin or mencord or mesar or olartan or olmeblo or olmec or olmes or olmetec or olpress or olsar or omesar or openvas or plaunac or santini or sarten or tensar or tensiol or vivactra or votum).tw.
98 pratosartan/ or (pratosartan or KT3‐671).tw.
99 saprisartan.mp.
100 tasosartan/ or (tasosartan or anazor or verdia).tw.
101 telmisartan/ or (telmisartan or actelsar or kinzal mono or kinzalmono or micardis or predxal or pritor or pritoral or semintra or tolura).tw.
102 valsartan/ or (valsartan or angiosan or cordinate or dalzad or diovan or diovane or kalpress or miten or nisis or prexxartan or provas or rixil or saval or tareg or tazea or troval or valpression or vals or valsocard or valtan or valtsu).tw.
103 zolasartan.mp.
104 or/83‐103
105 exp *renin inhibitor/
106 (RAS adj2 inhibit*).tw.
107 (renin adj2 inhibit*).tw.
108 aliskiren/ or (aliskiren or enviage or rasilez or riprazo or sprimeo or tekturna).tw.
109 ciprokiren.mp.
110 remikiren.mp.
111 terlakiren.mp.
112 zankiren.mp.
113 or/105‐112
114 exp *calcium channel blocking agent/
115 ((calcium adj2 antagonist*) or (calcium adj2 block*) or (calcium adj3 inhibit*)).tw.
116 exp amlodipine/ or (amlodipine or amdip or amloc or amlodipin or amlodipina or amlodis or amlopin or amlor or astudal or istin or levamlodipine or norvasc).tw.
117 amrinone/ or (amrinone or amcoral or amrinone or cardiotone or cartonic or cordemcura or inamrinone or inocor or vesistol or wincoram).tw.
118 aranidipine/ or (aranidipine or bec or sapresta).tw.
119 bencyclane/ or (bencyclane or bencyclan or benzcyclan or benzyclane or desoblit or dilangio compositu or fludilat or fluxema or halidor or ludilat).tw.
120 benidipine/ or (benidipine or coniel).tw.
121 bepridil/ or (bepridil or angopril or bedapin or bepadin or bepricol or cordium or cruor or unicordium or vascor).tw.
122 cilnidipine/ or (cilnidipine or atelec or cinaldipine or cinalong or siscard).tw.
123 cinnarizine/ or (cinnarizine or aplactan or aplexal or apomitere or apotomin or artate or carecin or cerebolan or cerepar or cibine or cimarizine or cinabioquim or cinaperazine or cinarizina or cinarizine or cinaziere or cinazyn or cinna or cinnabene or cinnacet or cinnaforte or cinnageron or cinnarazine or cinnarizin or cinnipirine or cinniprine or cisaken or corathiem or denapol or dimitron or dimitronal or eglen or giganten or glanil or hilactan or ixertol or katoseran or labyrin or lazeta or marisan or midronal or mitronal or olamin or processine or roin or sedatromin or sepan or siptazin or spaderizine or stugeron or stutgeron or stutgin).tw.
124 clentiazem/ or (clentiazem or logna).tw.
125 darodipine/ or (darodipine or dazodipine).tw.
126 diltiazem/ or (diltiazem or acalix or adizem or aldizem or altiazem or anginyl or angiodrox or angiotrofen or angiotrofin or angiozem or angitil or angizem or angoral or anoheal or anotrit or anzem or auscard or balcor or beatizem or blocalcin or britiazim or bruzem or calcicard or calnurs or cardcal or cardiazem or cardiben or cardiem or cardil or cardiosta or cardium or cardizem or carex or carreldon or cartia xt or cascor xl or cirilen or coras or cordizem or dazil or deltazen or diacor or diatal or diazem or dilacor or diladel or dilatam or dilatame or dilcard or dilcardia or dilem or dilfar or dilgard or diloc or dilren or dilrene or dilso or "dilt‐cd" or diltahexal or diltam or diltan or diltelan or diltia or diltiamax or diltiasyn or diltime or diltiwas or diltzac or dilzanton or dilzem or dilzene or dilzereal or dilzicardin or dinisor or doclis or dodexen or dyalac or entrydil or etizem or filazem or gadoserin or grifodilzem or hagen or helsibon or herben or herbesser or hesor or incoril or kaizem or kenzem or lacerol or levodex or levozem or lytelsen or masdil or miocardie or monotildiem or myonil or pazeadin or presoken or slozem or surazem or tazem or taztia or tiadil or tiamate or tiazac or tilazem or tildiem or tilker or vasmulax or vasocardol or wentizem or zandil or zatim or zemret or zemtrial or zildem or ziruvate).tw.
127 efonidipine/ or (efonidipine or finte or landel or elgodipine).tw.
128 etafenone/ or (etafenone or baxacor or dialicor or diethylaminoethoxyphenylpropiophenone hydrochloride or etafenon or etaphenone or kca or relicor).tw.
129 fantofarone.mp.
130 felodipine/ or (felodipine or agon or dilahex or dilofen or dilopin or fedil or felim or felo biochemie or felo er or felo‐basf or "felo puren" or felobal or felobeta or felocor or felodipin or felodur or felogamma or felogard or felop or felopine‐sr or fensel or flodil or hydac or keydipin or lodistad or modip or munobal or nirmadil or penedil or perfudal or plendil or preslow or prevex or renedil or selepine or splendil or versant).tw.
131 fendiline/ or (fendiline or cordan or digosensit or fendilar or phendiline or sensit or senzit).tw.
132 flunarizine/ or (flunarizine or flunarizine or sibelium).tw.
133 gallopamil/ or (gallopamil or elgiprona or galcan or gallobeta or gallopamile or methoxyverapamil or prebet or procorum).tw.
134 isradipine/ or (isradipine or dynacirc or icaz or isrodipine or lomir or prescal or vascal).tw.
135 lacidipine/ or (lacidipine or aponil or caldine or lacimen or lacipil or lacirex or lacitens or motens or viapres).tw.
136 lercanidipine/ or (lercanidipine or carmen or corifeo or lercadip or lercan or lerdip or masnidipine or zanedip or zanidip).tw.
137 lidoflazine/ or (lidoflazine or clinium or ordiflazine).tw.
138 lomerizine/ or (lomerizine or migsis or terranas).tw.
139 manidipine/ or (manidipine or artedil or calslot or franidipine or iperten or manyper or vascoman).tw.
140 mepirodipine/ or (mepirodipine or barnidipine or cyress or hypoca or libradin or vasexten).tw.
141 mibefradil/ or (mibefradil or "cerate 50" or posicor).tw.
142 nicardipine/ or (nicardipine or antagonil or barizine or bionicard or cardene or cardepine or cardibloc or cardipene or dacarel or dagan or flusemide or karden or lecibral or lincil or loxen or lucenfal or nerdipine or nicardal or nicardil or nicardipino or nicarpin or nicodel or nimicor or perdipina or perdipine or ranvil or ridene or roxen or rycarden or rydene or "saf card" or vasodin or vasonase).tw.
143 nifedipine/ or (nifedipine or adalat or cordipin or cordipine or corinfar or fenigidin or korinfar or nifangin or procardia or vascard).tw.
144 niguldipine.mp.
145 nivaldipine/ or (nilvadipine or escor or nilvadil or nivadil).tw.
146 nimodipine/ or (nimodipine or admon or brainal or calnit or eugerial or grifonimod or kenesil or kenzolol or modus or nidip or nimodilat or nimodipin or nimodipin‐isis or nimodipino or nimotop or nisom or nymalize or periplum or remontal or tropocer or vasoflex or vasotop).tw.
147 nisoldipine/ or (nisoldipine or angiolat or baymycard or corasol or nisoldin or sular or syscor).tw.
148 nitrendipine/ or (nitrendipine or balminil or baylotensin or bayotensin or baypresol or baypress or gericin or jutapress or lusopress or nidrel or niprina or nitre or nitregamma or nitren or nitrend ksk or nitrepress or nitre‐puren or nitrepuren or nitrendepat or nitrendi biochemie or nitrendidoc or nitrendimerck or nitrendipin or nitrendipin‐corax or nitrendipin‐ratiopharm or nitrendipincorax or nitrendipino or nitrendipinratiopharm or nitrensal or tensogradal or trendinol or vastensium).tw.
149 perhexiline/ or (perhexiline or perhexilene).tw.
150 prenylamine/ or (prenylamine or corontin or difril or segontin).tw.
151 semotiadil/ or (semotiadil or levosemotiadil or sesamodil).tw.
152 terodiline/ or (terodiline or biro or mictrol or micturin or terolin).tw.
153 tiapamil hydrochloride/ or (tiapamil or dimeditiapramine or larocord or thiapamil or verocainide or verocainine).tw.
154 verapamil/ or (verapamil or apo‐verap or apoacor or arpamyl or azupamil or berkatens or calan or calaptin or cardiagutt or cardibeltin or cardiolen or cardiovert or caveril or cintsu or civicor or "coer 24" or coraver or cordilat or cordilox or corpamil or covera or dexverapamil or dignover or dilacoran or dilacoron or durasoptin or falicard or finoptin or flamon or geangin or hexasoptin or ikacor or ikakor or ikapress or iproveratril or iso‐card sr or isoptin or isoptine or isoptino or izoptin or lekoptin or manidon or napamil or novapamyl or novo veramil or novopressan or phynoptin or quasar or ravamil or securon or univer or vasolan or vasomil or vasopten or verabeta or veracaps sr or veracor or verahexal or veraloc or veramex or veramil or verapin or verapress or veratad or verdilac or verelan or verexamil or veroptin stada or verpamil or vetrimil or vortac or zolvera).tw.
155 or/114‐154
156 exp *beta adrenergic receptor blocking agent/
157 ((beta adj2 adrenergic*) or (beta adj2 antagonist*)).tw.
158 ((beta adj2 block*) or (beta adj2 receptor*)).tw.
159 acebutolol/ or (acebutolol or acecor or acetobutolol or apoacebutolol or diasectral or espesil or flebutol or grifobutol or monitan or neptal or neptall or novoacebutolol or prent or rhotral or sectral).tw.
160 alprenolol/ or (alprenolol or alfeprol or alloprenalol or alpheprol or alprendol or alprenol or alprenololum or apliobal or aprenolol or aptia or aptin or aptin‐duriles or aptinduriles or aptina or aptine or aptol or astra or betacard or betapin).tw.
161 amosulalol/ or (amosulalol or amsolulol or howgan or lowgan).tw.
162 arotinolol/ or (arotinolol or almarl).tw.
163 atenolol/ or (atenolol or ablok or adoll or alonet or altol or anolene or anolpin or anselol or arandin or asten or atarox or atcardil or atecard or atehexal or atelol or atenblock or atendol or atenet or ateni or atenil or ateno or atenogamma or atenol or atereal or aterol or atestad or atinol or atolmin or b‐vasc or betablok or betacar or betarol or betatop ge or beten or bloket or blokium or blotex or cardioten or catenol or coratol or corotenol or durabeta or esatenolol or evitocor or farnormin or felo‐bits or hypernol or hypoten or internolol or "lo‐ten" or loten or lotenal or martenol or mirobect or myocord or neotenol or nolol or normalol or normaten or normiten or nortelol or noten or oraday or ormidol or paesumex or plenacor or preloc or premorine or prenolol or prenormine or ranlol or rozamin or serten or stermin or temoret or tenblok or tenidon or tenoblock or tenocor or tenol or tenolin or tenolol or tenopress or tenoprin or tenormin or tenormine or tenostat or tensig or tensinor or ternolol or therabloc or tredol or vascoten or velorin or vericordin or wesipin).tw.
164 befunolol/ or (befunolol or befnolol or benfuran or bentos or bentox or glauconex or glaukonex).tw.
165 betaxolol/ or (betaxolol or "alcon betoptic" or betac or betarun or betasel or betaxon or betoptic or betoptima or betoquin or kerlon or kerlone or kerlong or levobetaxolol or lokren or optibet or optipress or oxodal or tonobexol).tw.
166 bevantolol/ or (bevantolol or benatol or bevantololum or calvan or ranestol or vantol).tw.
167 bisoprolol/ or (bisoprolol or concor).tw.
168 bopindolol/ or (bopindolol or sandonorm or wandonorm).tw.
169 bucindolol.mp.
170 bucumolol/ or (bucumolol or bucumarol or bukumolol).tw.
171 bufuralol/ or (bufarolol or angium).tw.
172 bufetolol/ or (bufetolol or adbiol).tw.
173 bunitrolol/ or (bunitrolol or betrilol or stresson or tilcant).tw.
174 butofilolol.mp.
175 carazolol/ or (carazolol or conducton or suacron).tw.
176 carteolol/ or (carteolol or arteolol or arteoptic or arteoptik or caltamol or calte or carbonolol or carteabak or carteol or cartrol or elebloc or endak or glauteolol or karol or karteol or mikelan or ocupress or stobol or teoptic).tw.
177 carvedilol/ or (carvedilol or cardiol or cardivas or carvedlol or carvipress or carvrol or coreg or dilatrend or dilbloc or dimitone or eucardic or kredex or quarto or quarto).tw.
178 celiprolol/ or (celiprolol or abecor or celectol or dilanorm or selecor or selectol).tw.
179 cetamolol/ or (cetamolol or betacor).tw.
180 cloranolol/ or (cloranolol or tobanum).tw.
181 epanolol/ or (epanolol or visacor).tw.
182 esmolol/ or (esmolol or brevibloc or miniblock).tw.
183 indenolol/ or (indenolol or pulsan).tw.
184 labetalol/ or (labetalol or abetol or albetol or amipress or apolabetalol or biascor or dilevalol or dilevalon or hybloc or ibidomide or ipolab or labelol or labesine or lamitol or levadil or liondox or normodyne or presdate or presolol or pressalolo or salmagne or trandate or unicard).tw.
185 levobunolol/ or (levobunolol or ak beta or akbeta or apolevobunolol or betagan or betasite or bunolgan or gotensin or l bunolol hydrochloride or levo bunolol or novolevobunolol or pmslevobunolol or ultracortenol or vistagan or vistagen).tw.
186 mepindolol/ or (mepindolol or betagon or caridian or corindolan or "she 222").tw.
187 metipranolol/ or (metipranolol or beta ophtiole or betalol or betamann or betamet or betanol or betanolol or disorat or glauline or methypranol or normoglaucon mite or ophtiole or optipranolol or trimepranol).tw.
188 metoprolol/ or (metoprolol or "beloc duriles" or "belok zok" or betaloc or betalok or lopressor or seloken or spesicor or spesikor or toprol).tw.
189 moprolol.mp.
190 nadolol/ or (nadolol or betadol or corgard or farmagard or nadic or solgol or solgol).tw.
191 nadoxolol.mp.
192 nebivolol/ or (nebivolol or bivolet or bystolic or dexnebivolol or levonebivolol or lobivon or narbivolol or nebilet or nebilox or silostar).tw.
193 nifenalol/ or (nifenalol or "d inpea" or "dextro inpea" or dextro levo inpea or inpea or isophenethanol or isophenethyl alcohol or isopropylaminonitrophenylethanol or nefenalol).tw.
194 oxprenolol/ or (oxprenolol or cordexol or coretal or koretal or oxtrenolol or tevacor or trasacor or trasicor).tw.
195 pafenolol.mp.
196 penbutolol/ or (penbutolol or betapresin or betapressin or blocotin or levatolor or paginol).tw.
197 pindolol/ or (pindolol or barbloc or betapindol or blocklin or calvisken or carvisken or decreten or dranolis or durapindol or hexapindol or hydroxypropylaminopropoxyindol or lb46 or nonspi or novo‐pindol or pectobloc or pectoblock mite or pidol or pinbetol or pinden or pindol or pindomex or pindoreal or pinloc or pinolol or pinsken or prindolol or prinodolol or pyndale or treparasen or viskeen or visken or viskene or vypen).tw.
198 practolol/ or (practolol or dalzic or eraldin or practolole or praktolol or praktololu or proctalol or teranol).tw.
199 pronetalol/ or (pronetalol or alderlin or nethalide or pronetal or pronethalol).tw.
200 propanolol/ or (propranolol or acifol or adrexan or alperol or anaprilin or anapriline or anaprilinium or anapryline or angilol or apsolol or arcablock or artensol or authus or avlocardyl or becardin or bedranol or beprane or bercolol or berkolol or beta neg or beta tablinen or beta timelets or betabloc or betadipresan or betadren or betaneg or betaprol or betares or betaryl or blocard or blocaryl or cardinol or ciplar or corbeta or deralin or dexpropranolol or dibudinate or dideral or dociton or docitone or durabeton or duranol or efektolol or elbrol or emforal or farmadral or farprolol or frekven or frina or hemangeol or hemangiol or hopranolol or ikopal or Impral or Inderal or inderalici or inderex or indicardin or indobloc or innopran or inpanol or ipran or lederpronol or levopropranolol or napriline or noloten or obsidan or obsin or obzidan or oposim or phanerol or prandol or prano puren or pranopuren or prestoral or prolol or pronovan or propabloc or propal or propalong or propayerst or propercuten or prophylux or propra ratiopharm or propral or propranur or proprasylyt or proprasylyte or reducor or rexigen or sagittol or stapranolol or sumial or tenomal or tensiflex or waucoton).tw.
201 sotalol/ or (sotalol or alosot or beta‐cardone or betacardone or betades or betapace or bonpro or corsotalol or darob or dexsotalol or dextrosotalol or favorex or gilucor or hipecor or isotalol or jutalex or levosotalol or rentibloc or rotalol or satalol or satolol or solavert or sorine or sota saar or sotab or sotabeta or sotacol or sotacor or sotahexal or sotalex or sotaper or sotapor or sotastad or sotylize or tachytalol).tw.
202 sulfinalol.mp.
203 talinolol/ or (talinolol or cordanum).tw.
204 tertatolol/ or (tertatolol or artex or prenalex).tw.
205 tilisolol/ or (tilisolol or daim or selecal).tw.
206 timolol/ or (timolol or apotimolol or blocadren or optimal or timacar or timolo or timoptic or timoptol or titol).tw.
207 toliprolol/ or (toliprolol or doberol).tw.
208 xibenolol.mp.
209 or/156‐208
210 exp *alpha adrenergic receptor blocking agent/
211 ((adrenergic adj2 alpha) or (adrenergic adj2 antagonist*)).tw.
212 ((adrenergic adj2 block*) or (alpha adj2 block*) or (receptor* adj2 block*)).tw.
213 (alpha adj2 receptor antagonist*).tw.
214 alfuzosin/ or (alfuzosin* or alcinin or alfabax or alfetim or alfoten or alfu or alfudex or alfugen or alfukal or alfulek or alfunar or alfural or alfuran or alfuro or alfusin or alfuzin or alfuzostad or alfuzozin or altofen or alugen or alzmeran or azosin or benestan or besavar or dalfaz or danafusin or faralzin or flotral or fuzatal or lafunomyl or mittoval or ofuxal or prostazosin or rilif or taurazil or unibenestan or urion or urobene or uroxatral or urozosin or vasran or xatger or xatral or zochek or zoprost).tw.
215 bunazosin/ or (bunazosin or bunatenon or bunazocine or detantol).tw.
216 doxazosin/ or (doxazosin* or alfamedin or cardular or cardura or carduran or diblocin or doxa puren or doxauro or doxacor or doxagamma or doxamax or doxatensa or doxazomerck or doxazosina or jutalar or progandol or uriduct or uroprost or zoxan).tw.
217 metazosin/ or (metazosin or kenosin).tw.
218 neldazosin/ or neldazosin.tw.
219 prazosin/ or (prazosin or adversuten or alpress or atodel or decliten or deprazolin or duramipress or eurex or furazosin or hexapress or hypotens or hypovase or hyprosin or lentopres or minipres or minesin or minipres or minipress or minison or mizosin or patsolin or peripress or prasig or pratisol or pratsiol or prazac or prazopress or prazosine or pressin or prozosin).tw.
220 silodosin/ or (silodosin or rapaflo or silodyx or urief or urorec).tw.
221 tamsulosin/ or (tamsulosin or alna or amsulosin or flomax or flomaxtra or hamal or josir or mapelor or mecir or omexel or omic or omix or omnic or pradif or secotex or tamfrex or urolosin).tw.
222 terazosin/ or (adecur or conmy or deflox or dysalfa or flotrin or heitrin or hitrin or hydrin or hytracin or hytrin or hytrine or hytrinex or itrin or kinzosin or magnurol or olyster or sinalfa or teradrin or terafluss or teralfa or terapam or teraprost or terasin or terazosab or urodie or uroflo or uroprost or vasocard or vasomet or vicard).tw.
223 tiodazosin/ or (tiodazosin or thiodazosin).tw.
224 trimazosin/ or (trimazosin or cardovar).tw.
225 or/210‐224
226 (45 and 82) or (45 and 104) or (45 and 113) or (45 and 155) or (45 and 209) or (45 and 225) or (82 and 104) or (82 and 113) or (82 and 155) or (82 and 209) or (82 and 225) or (104 and 113) or (104 and 155) or (104 and 209) or (104 and 225) or (113 and 155) or (113 and 209) or (113 and 225) or (155 and 209) or (155 and 225) or (209 and 225)
227 hypertension/
228 hypertens*.tw.
229 ((chang* or elevat* or high or lower* or rais*) adj2 (arterial pressur* or blood pressur* or diastolic pressur* or systolic pressur*)).tw.
230 ((chang* or elevat* or high or lower* or rais*) adj2 (bp or dbp or sbp)).tw.
231 or/227‐230
232 randomized controlled trial/
233 crossover procedure/
234 double‐blind procedure/
235 (randomi* or randomly).tw.
236 (crossover* or cross‐over*).tw.
237 placebo.ab.
238 (doubl* adj blind*).tw.
239 assign*.ab.
240 allocat*.ab.
241 or/232‐240
242 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
243 maternal hypertension/ or exp pulmonary hypertension/ or exp portal hypertension/ or intracranial hypertension/ or renovascular hypertension/ or intraocular hypertension/
244 (anaesth* or anesth* or ante natal* or antenatal* or bleeding or caesarean or cesarean or caesarian or cesarian or caesarien or cesarien or cirrho* or eclamp* or epidural or fetal or foetal or fetus or foetus or gestational hypertens* or glaucom* or interocular or intraocular or intracran* or intuba* or lactation* or neonat* or new born* or newborn* or obstetr* or ocular or ophthalm* or peri partum or peripartum or portal hypertens* or portopulmonary or pre eclamp* or preeclamp* or pregnancies or pregnancy or pregnant or pre natal* or prenatal* or pulmonary hypertens* or pulmonary arterial hypertens* or secondary hypertens* or spinal).ti.
245 241 not (242 or 243 or 244)
246 226 and 231 and 245
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov Search Date: 12 March 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Condition or disease: Hypertension NOT (Pulmonary Hypertension OR Ocular Hypertension OR Portal Hypertension) Other terms: randomized Study type: Interventional Studies (Clinical Trials) Intervention/treatment: combination therapy AND monotherapy Study Results: All Studies OR Condition or disease: Hypertension NOT (Pulmonary Hypertension OR Ocular Hypertension OR Portal Hypertension) Other terms: randomized Study type: Interventional Studies (Clinical Trials) Intervention/treatment: combination therapy AND versus Study Results: All Studies OR Condition or disease: Hypertension NOT (Pulmonary Hypertension OR Ocular Hypertension OR Portal Hypertension) Other terms: randomized Study type: Interventional Studies (Clinical Trials) Intervention/treatment: combination therapy AND vs Study Results: All Studies
Appendix 2. Pharmaceutical companies checked
| Company | Website |
| Abbott | www.abbott.com/policies/clinical-trials.html [Search Date: 10 August 2018] |
| AbbVie | www.abbvie.com/our-science/clinical-trials/conduct-of-clinical-trials.html [Search Date: 10 August 2018] |
| AstraZeneca | www.astrazenecaclinicaltrials.com/ [Search Date: 10 August 2018] |
| Bayer | pharma.bayer.com/en/research-and-development/clinical-trials/trial-finder/index.php [Search Date: 10 August 2018] |
| Boehringer Ingelheim | trials.boehringer-ingelheim.com/trial_results.html [Search Date: 10 August 2018] |
| Bristol‐Myers Squibb | www.bms.com/clinical_trials/Pages/home.aspx [Search Date: 10 August 2018] |
| Daiichi Sankyo | www.daiichisankyo.com/rd/our_approach/clinical_studies/index.html [Search Date: 10 August 2018] |
| GlaxoSmithKline | www.gsk-clinicalstudyregister.com/ [Search Date: 10 August 2018] |
| Menarini | www.menarini.it/Home/Innovazione-Ricerca/Studi-Clinici [Search Date: 10 August 2018] |
| Merck | www.merck.com/clinical-trials/search.html [Search Date: 12 August 2018] |
| Mylan | www.mylanpharms.com/ [Search Date: 12 August 2018] |
| Novartis | www.novctrd.com/CtrdWeb/searchbyindication.nov [Search Date: 12 August 2018] |
| Pfizer | www.pfizer.com/research/research_clinical_trials/trial_results_research_progress [Search Date: 12 August 2018] |
| Recordati | www.recordati.com/en/pharmaceutical_operations/r_and_d/pubblications [Search Date: 12 August 1018] |
| Sanofi Aventis | en.sanofi.com/rd/clinical_trials/our_commitments/clinical_study_results.aspx [Search Date: 12 August 2018] |
| Servier | clinicaltrials.servier.com/find-clinical-trials/ [Search Date: 12 August 2018] |
| Takeda | www.takedaclinicaltrials.com [Search Date: 12 August 2018] |
| Teva | www.tevapharm.com/ [Search Date: 12 August 2018] |
| UCB | www.ucb.com/our-science/Our-clinical-studies [Search Date: 12 August 2018] |
| In recent years, pharmaceutical companies have referred to the trial registry at ClinicalTrials.gov, which is included in the literature search. | |
Appendix 3. Reviews and guidelines checked
Hilleman 1999; Ruzicka 2001; Law 2003a; Law 2009; Wald 2009; Gradman 2010; Hopkins 2010; Lv 2010; Norris 2010; Sood 2010; Liu 2013; Makani 2013; Bakris 2014; JNC 8 2014; Povoa 2014; Fu 2017; He 2017; ESH/ESC 2018; Reboussin 2018; Whelton 2018; NICE 2019; Hypertension Canada 2020.
Data and analyses
Comparison 1. Combination therapy versus monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total mortality | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.08, 21.72] |
| 1.1.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.08, 21.72] |
| 1.1.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.2 Serious adverse events | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.31, 1.92] |
| 1.2.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.24, 1.64] |
| 1.2.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.34, 29.42] |
| 1.3 Cardiovascular events | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.22, 4.41] |
| 1.3.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.10, 3.95] |
| 1.3.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.13, 75.69] |
| 1.4 Cardiovascular mortality | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 Withdrawals due to adverse effects | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
| 1.5.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.49, 1.35] |
| 1.5.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.45] |
| 1.6 Reaching blood pressure control | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 People with diabetes, target ≤ 120/80 mmHg | 1 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.18] |
| 1.6.2 People with diabetes, target ≤ 140/90 mmHg | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.24, 3.22] |
| 1.6.3 People without diabetes, target ≤ 140/90 mmHg | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.62, 1.28] |
| 1.7 Systolic blood pressure change from baseline at end of 1 year | 3 | 548 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐5.39, 1.27] |
| 1.7.1 People with diabetes | 2 | 419 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐8.27, 3.19] |
| 1.7.2 People without diabetes | 1 | 129 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐7.28, 2.62] |
| 1.8 Diastolic blood pressure change from baseline at end of 1 year | 2 | 443 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.21, 0.96] |
| 1.8.1 People with diabetes | 1 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.56, 0.78] |
| 1.8.2 People without diabetes | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.45 [‐1.40, 4.30] |
Comparison 2. Combination therapy versus monotherapy (men versus women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Women | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.52, 3.00] |
| 2.1.2 Men | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| 2.2 Withdrawals due to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 Women | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.43, 3.73] |
| 2.2.2 Men | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.66] |
| 2.3 Systolic blood pressure change from baseline at end of 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Women | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [‐2.10, 5.58] |
| 2.3.2 Men | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐3.25, 1.19] |
| 2.4 Diastolic blood pressure change from baseline at end of 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Women | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐1.96, 2.90] |
| 2.4.2 Men | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐2.08, 0.54] |
Comparison 3. Combination with potassium‐sparing diuretics versus monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Total mortality | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.87] |
| 3.2 Serious adverse events | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.10, 5.04] |
| 3.3 Cardiovascular events | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.13, 15.71] |
| 3.4 Cardiovascular mortality | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5 Withdrawals due to adverse effects | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.6 Reaching blood pressure control | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BENEDICT‐A 2004.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind trial Follow‐up: 36 months |
|
| Participants | Inclusion criteria: aged ≥ 40 years with hypertension (defined as an untreated systolic blood pressure ≥ 130 mmHg or a diastolic blood pressure ≥ 85 mmHg), history of type 2 diabetes mellitus not exceeding 25 years, urinary albumin excretion rate < 20 μg/min, and serum creatinine concentration ≤ 1.5 mg/dL Exclusion criteria: HbA1c > 11%, non‐diabetic renal disease, heart failure, or specific indications or contraindications to ACEI or CCB therapy Country: Italy |
|
| Interventions | Monotherapy 1: verapamil SR 240 mg daily Monotherapy 2: trandolapril 2 mg daily Combination therapy: verapamil 180 mg + trandolapril 2 mg daily Target blood pressure 120/80 mmHg. Additional antihypertensive drugs were allowed to achieve the target blood pressure in the following steps: step 1, hydrochlorothiazide or furosemide; step 2, doxazosin, prazosin, clonidine, methyldopa, or beta‐blockers (allowed based on specific indications); and step 3, minoxidil or long‐acting dihydropyridine CCB. Potassium‐sparing diuretics, inhibitors of the renin‐angiotensin system, and non‐dihydropyridine CCBs different from the study drugs were not allowed |
|
| Outcomes | Primary endpoint: development of persistent microalbuminuria (urinary albumin excretion ≥ 20 μg/min at 2 consecutive visits) Other outcomes: urinary albumin excretion, blood pressure after 1 month, major cardiovascular events, overall and cardiovascular mortality, HbA1c, retinal changes, adverse effects and safety laboratory parameters |
|
| Funding sources | Abbott GmbH & Co | |
| Declarations of interest | Not reported | |
| Notes | Trial started March 1997. We used data of participants without previous antihypertensive treatment (verapamil + trandolapril: 115 participants, verapamil: 106 participants, trandolapril: 109 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to each therapy in a 1:1 ratio according to a computer‐generated randomisation list created by the Biometric Unit of Abbott |
| Allocation concealment (selection bias) | Low risk | The participant randomisation number was requested by telephone or fax and was assigned by the Treatment Assignment Secretariat at the Mario Negri Institute (Ranica, Italy) by an independent investigator unaware of treatments' allocation schemes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study treatments were externally non‐distinguishable pink‐ivory, 2‐coloured capsules. Investigators, participants, care providers, endpoint evaluators, monitors, and data analysts were masked throughout the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators, participants, care providers, endpoint evaluators, monitors, and data analysts were masked throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Schematic diagram of the trial |
| Selective reporting (reporting bias) | Low risk | Protocol available. Individual participant data provided |
| Other bias | Unclear risk | Inclusion criteria were changed during the trial (from untreated blood pressure ≥ 140/90 mmHg to ≥ 130/85 mmHg). Blood pressure targets were also changed from 130/85 mmHg to 120/80 mmHg (protocol amendment 3; 27 May 1999). Subgroup of participants naïve to antihypertensives not predefined. Study not designed for our objectives |
PREMIER 2003.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind trial Follow‐up: 52 weeks |
|
| Participants | Inclusion criteria: aged 40 to 75 years with type 2 diabetes, hypertension defined as supine systolic blood pressure ≥ 140 mmHg and < 180 mmHg and supine diastolic blood pressure < 110 mmHg, and albumin excretion rate ≥ 20 μg/min and < 500 μg/min in at least 2 of 3 assays Exclusion criteria: HbA1c ≥ 9% during the 3 months before the study, with presumed non‐diabetic kidney disease, serum creatinine ≥ 140 μmol/L, known contraindications to ACEI therapy or indapamide, or other severe disease Countries: Argentina, Austria, Belgium, Brazil, the Czech Republic, France, Germany, Hungary, Ireland, Mexico, Morocco, the Netherlands, Poland, Slovakia, South Africa, Spain, Switzerland, Tunisia, Turkey, the UK |
|
| Interventions | Both groups: open 4‐week pre‐randomisation run‐in period of placebo once daily Monotherapy: enalapril 10 mg daily Combination therapy: perindopril 2 mg + indapamide 0.625 mg once daily Target blood pressure was < 140/90 mmHg. Dose adjustment was permitted after week 12 in double‐blind steps: perindopril 4 mg + indapamide 1.25 mg or enalapril 20 mg then perindopril 8 mg + indapamide 2.5 mg or enalapril 40 mg. Non‐study antihypertensive drugs were not permitted. |
|
| Outcomes | Primary outcome: change in the albumin excretion rate after 1 year Secondary outcomes: albumin/creatinine ratio, supine blood pressure and blood pressure response defined as a reduction in systolic blood pressure < 140 mmHg and diastolic blood pressure < 90 mmHg or reduction of systolic blood pressure ≥ 20 mmHg or reduction of diastolic blood pressure ≥ 10 mmHg, or a combination of these. Serious adverse events were predefined as those that were fatal or required prolonged hospitalisation. |
|
| Funding sources | Institut de Recherches Internationales Servier | |
| Declarations of interest | Not reported | |
| Notes | Trial conducted between March 1997 and January 2001. The trial recruited 481 participants, and we used data of 109 participants without previous antihypertensive treatment (perindopril + indapamide: 55 participants; enalapril: 54 participants). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation method used to assign treatments (personal communication) |
| Allocation concealment (selection bias) | Low risk | At the beginning of the study, investigators received randomised permutation blocks and the corresponding sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study products were supplied in the form of capsules of identical appearance. Prior to the study, the investigator received the therapeutic units and the corresponding coded envelopes. Blister packs and boxes were identified with a unique drug code number for each participant. A 2‐part tear‐off label was affixed to each blister pack and box. When the medication was delivered to the participant, the investigator removed the tear‐off portion of the label and attached it to the participant's case report form |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators provided description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In our subgroup, there were more withdrawals due to lack of efficacy in the monotherapy group (6 with monotherapy versus 0 with combination therapy) |
| Selective reporting (reporting bias) | Low risk | Investigators provided results data as requested |
| Other bias | Unclear risk | Subgroup of participants naïve to antihypertensives not predefined. Study not designed for our objectives |
PREVER‐treatment 2016.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, clinical trial Follow‐up: 18 months |
|
| Participants | Aged 30 to 70 years, stage 1 hypertension (140 mmHg to 159 mmHg/90 mmHg to 99 mmHg; > 130 mmHg in people with diabetes), no current use of blood pressure‐lowering medication, no previous cardiovascular disease Country: Brazil |
|
| Interventions | Monotherapy: losartan starting dose 50 mg, up to 100 mg daily Combination therapy: chlorthalidone 12.5 mg + amiloride 2.5 mg starting dose up to chlorthalidone 25 mg + amiloride 5 mg in the same pill daily Amlodipine up to 10 mg daily and propranolol up to 160 mg daily, in an open fashion, to be added if blood pressure not controlled (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) |
|
| Outcomes | Primary: blood pressure variation and proportion of use of add‐on drugs, adverse events, development or worsening of microalbuminuria, and left ventricular hypertrophy on electrocardiogram Secondary: fatal or major cardiovascular events: myocardial infarction, stroke, coronary interventions, heart failure, duplication of creatinine |
|
| Funding sources | Department of Science and Technology (DECIT), Health Ministry; National Council of Research (CNPq) and Agency for Funding of Studies and Projects (FINEP), Science and Technology Ministry; National Institute of Health Technology Assessment (IATS); and Funding of Incentive to Research (FIPE), Hospital de Clınicas de Porto Alegre, all in Brazil | |
| Declarations of interest | All authors reported no conflicts of interest and financial disclosures with regard to the subject of the manuscript. | |
| Notes | Trial conducted between February 2011 and September 2016. The trial recruited 655 participants, and we used data from 200 participants without previous antihypertensive treatment (chlorthalidone + amiloride: 116 participants; losartan: 84 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computer‐generated list, using validated software (Random Allocator), with variable block sizes of 4, 6, 8, or 10 and was stratified by centre |
| Allocation concealment (selection bias) | Low risk | To guarantee concealment of the allocation list, randomisation was implemented through a 24‐hour web‐based automated system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The 2 study drugs were identical in size, shape, colour, taste, and texture |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, members of the steering committee, healthcare staff, data collectors, and outcome assessors but no members from the data safety monitoring committee were blinded as to whether participants had received chlorthalidone/amiloride or losartan |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of incomplete follow‐up is balanced between groups |
| Selective reporting (reporting bias) | Low risk | Investigators provided results data as requested |
| Other bias | Unclear risk | Subgroup of participants naive to antihypertensives not predefined. Study not designed for our objectives |
REASON 2001.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind trial Follow‐up: 12 months |
|
| Participants | Inclusion criteria: aged 18 to 84 years with essential hypertension defined as a supine systolic blood pressure ≥ 160 mmHg and < 210 mmHg, or a supine diastolic blood pressure ≥ 95 mmHg and < 110 mmHg, or both. In all cases, hypertension was uncomplicated. Exclusion criteria: people receiving medication for diabetes, hypocholesteraemia, or cardiovascular disease Countries: Australia, Austria, Belgium, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden, Switzerland, the UK |
|
| Interventions | Both groups: 4‐week placebo period Monotherapy: atenolol 50 mg Combination therapy: perindopril 2 mg + indapamide 0.625 mg In both groups, the medication was taken orally in the morning as a single dose. The dosage was then adapted to the blood pressure, and the dose was doubled (2 capsules once daily) after 3 months if systolic blood pressure remained > 160 mmHg or diastolic blood pressure > 90 mmHg, or both. At the end of the procedure, drug dosage was progressively decreased over 8 to 15 days to avoid any complication caused by atenolol withdrawal |
|
| Outcomes | Brachial systolic blood pressure, diastolic blood pressure, pulse pressure, aortic pulse wave velocity, carotid and aortic blood pressures, heart rate, adverse effects Target blood pressure defined as < 140/90 mmHg |
|
| Funding sources | INSERM, Association Claude Bernard, GPH‐CV, and Laboratoires Servier | |
| Declarations of interest | Not reported | |
| Notes | Study dates not reported. Trial recruited 471 participants. We used data from 129 participants without previous antihypertensive treatment (perindopril/indapamide: 63 participants; atenolol: 66 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation method used to assign treatments (personal communication) |
| Allocation concealment (selection bias) | Low risk | Prior to the study, the investigator received the therapeutic units and the corresponding coded envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study products were supplied in the form of capsules of identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All measurements were analysed by 2 physicians blinded to treatment, clinical data, and physical examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the entire study, 471 participants were randomised and 354 completed active treatment period, but reasons for withdrawals were provided for only 96 participants. Information lacking on 7 participants in the perindopril + indapamide group and 12 participants in the atenolol group |
| Selective reporting (reporting bias) | Low risk | Investigators provided results data as requested |
| Other bias | Unclear risk | Subgroup of participants naïve to antihypertensives not predefined. Study not designed for our objectives |
ACEI: angiotensin‐converting enzyme inhibitor; CCB: calcium channel blocker; HbA1c: glycated haemoglobin; SR: slow release.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACCELERATE 2011 | Follow‐up only 32 weeks |
| BENEDICT‐B 2011 | We requested data for participants naïve to antihypertensive drugs. The authors provided individual participant data, but there were fewer than 50 participants per group (trandolapril: 39 participants, trandolapril + verapamil: 40 participants) |
| DEMAND 2011 | We requested data for participants naïve to antihypertensive drugs. The authors provided individual participant data, but there were fewer than 50 participants per group (delapril: 33 participants, delapril + manidipine: 38 participants) |
| MRC‐O 1992 | Single‐blind trial |
| ONTARGET 2008 | Participants entered a run‐in period in which they received ramipril 2.5 mg once daily for 3 days, followed by telmisartan 40 mg + ramipril 2.5 mg once daily for 7 days and then ramipril 5 mg + telmisartan 40 mg for 11 to 18 days, so participants were not naïve to antihypertensive treatment at randomisation |
| PATHWAY‐1 2017 | At week 17 all participants received forced open‐label combination, so the double‐blind comparison of monotherapy versus combination lasted only 16 weeks |
| PICXEL 2005 | We requested data for participants naïve to antihypertensive drugs. The authors provided aggregate data, but there were fewer than 50 participants per group (enalapril: 46 participants, perindopril + indapamide: 40 participants) |
| PREVER‐prevention 2016 | The participants have pre‐hypertension, a condition not within the scope of this review |
| Zhang 2010 | Not stated if this was a double‐blind trial. No data for any of our primary outcomes |
Characteristics of studies awaiting classification [ordered by study ID]
Derosa 2013.
| Methods | Multicentre, randomised, double‐blind, clinical trial Follow‐up: 12 months |
| Participants | Inclusion criteria: aged ≥ 18 with stage 1 essential hypertension (defined as sitting systolic blood pressure ≥ 140 mmHg and < 160 mmHg and sitting diastolic blood pressure ≥ 90 mmHg and < 100 mmHg after a 2‐week wash‐out placebo period) Exclusion criteria: type 2 diabetes mellitus, impaired liver or kidney function, anaemia, unstable cardiovascular conditions (e.g. New York Heart Association (NYHA) class I to IV congestive heart failure or a history of myocardial infarction or stroke) or cerebrovascular conditions within 6 months of study enrolment Country: Italy |
| Interventions | Monotherapy 1: olmesartan 20 mg Monotherapy 2: amlodipine 10 mg Combination therapy: olmesartan 20 mg + amlodipine 5 mg in single tablet |
| Outcomes | Body weight, body mass index, systolic and diastolic blood pressures, fasting plasma glucose, fasting plasma insulin, lipid profile, tumour necrosis factor‐α, retinol binding protein‐4, and interleukins 6 and 7 At baseline, and after 6 and 12 months, participants underwent a euglycaemic, hyperinsulinaemic clamp |
| Notes | We requested data for outcomes of interest in the subgroup of participants naïve to antihypertensive treatment but received no response There are 6 publications of the trial with the same data; as of August 2019, 5 of them have been retracted |
Derosa 2014.
| Methods | Multicentre, randomised, double‐blind, clinical trial Follow‐up: 24 months |
| Participants | Inclusion criteria: outpatients aged < 65 years, with a first‐diagnosed essential hypertension (diastolic blood pressure > 90 mmHg and < 110 mmHg or systolic blood pressure > 140 mmHg and < 180 mmHg, or both) and naïve to antihypertensive treatment Exclusion criteria: hypertrophic cardiomyopathies due to aetiologies other than hypertension; history of heart failure, left ventricular ejection fraction ≤ 50%, angina, stroke, transient ischaemic cerebral attack, coronary artery bypass surgery, or myocardial infarction any time prior to first visit; concurrent symptomatic arrhythmia; liver dysfunction; creatinine > 1.5 mg/dL; endocrine, infective, or inflammatory disorders; use of anti‐inflammatory medications Country: Italy |
| Interventions | Monotherapy 1: enalapril 20 mg once a day Monotherapy 2: lercanidipine 10 mg once a day Combination therapy: enalapril 20 mg + lercanidipine 10 mg once a day |
| Outcomes | Body mass index, systolic and diastolic blood pressure, fasting plasma glucose, lipid profile, lipoprotein a, soluble receptor for advanced glycation end products, soluble CD40 ligand, serum myeloperoxidase, high sensitivity C‐reactive protein and tumour necrosis factor‐α |
| Notes | We requested data for outcomes of interest but received no response. There are 2 publications of the trial with the same data; as of August 2019, 1 of them has been retracted |
Derosa 2016.
| Methods | Randomised, double‐blind, clinical trial Follow‐up: 12 months |
| Participants | Inclusion criteria: outpatients aged ≥ 18, overweight, mild to moderate primary hypertension (systolic blood pressure ≥ 140 mmHg and < 180 mmHg and/or diastolic blood pressure ≥ 90 mmHg and < 105 mmHg), well‐controlled type 2 diabetes mellitus (HbA1c ≤ 7.5%), low‐density lipoprotein cholesterol < 160 mg/dL Exclusion criteria: hypertrophic cardiomyopathies due to aetiologies other than hypertension, history of heart failure, history of angina, stroke, transient ischaemic cerebral attack, coronary artery bypass surgery, myocardial infarction, concurrent known symptomatic arrhythmia; liver dysfunction (aspartate aminotransferase or alanine aminotransferase exceeding 2‐fold the upper limit); creatinine > 1.5 mg/dL, hypersensitivity to the study drugs, pregnant women and women of childbearing potential Country: Italy |
| Interventions | Monotherapy 1: olmesartan 20 mg daily Monotherapy 2: amlodipine 10 mg daily Combination therapy: olmesartan 20 mg + amlodipine 5 mg in single tablet daily |
| Outcomes | Systolic and diastolic blood pressure, lipoprotein (a), myeloperoxidase, isoprostanes, and PON‐1 |
| Notes | We requested data for outcomes of interest in the subgroup of participants naïve to antihypertensive treatment but received no response. |
INSIGHT 2000.
| Methods | Multicentre, randomised, double‐blind, clinical trial Mean follow‐up: 3.5 years |
| Participants | Inclusion criteria: aged 55 to 80 years with hypertension (blood pressure ≥ 150/95 mmHg or ≥ 160 mmHg systolic) and at least 1 additional cardiovascular risk factor: hypercholesterolaemia; smoker (10 cigarettes per day currently or up to 1 year before entry); family history of myocardial infarction in parent or sibling before age 50 years; current left‐ventricular hypertrophy, coronary heart disease; left‐ventricular strain; peripheral vascular disease Countries: Denmark, France, Israel, Italy, the Netherlands, Norway, Spain, Sweden, the UK |
| Interventions | Monotherapy: initially nifedipine 30 mg daily Combination therapy: hydrochlorothiazide 25 mg + amiloride 2.5 mg daily Dose titration was by dose doubling, and addition of atenolol 25 to 50 mg or enalapril 5 to 10 mg in people whose blood pressure fell by < 20/10 mmHg or was > 140/90 mmHg |
| Outcomes | Primary: cardiovascular death, myocardial infarction, heart failure or stroke Secondary: total mortality; death from a vascular cause; and non‐fatal vascular events including transient ischaemic attacks, angina (new or worsening), and renal failure; serious adverse events |
| Notes | We requested data for outcomes of interest in the subgroup of participants without previous antihypertensive treatment but received no response |
Maeda 2009.
| Methods | Randomised, double‐blind, clinical trial Follow‐up: 24 months |
| Participants | Hypertensive outpatients in sinus rhythm |
| Interventions | Monotherapy 1: telmisartan 20 mg to 80 mg Monotherapy 2: amlodipine 2.5 mg to 10 mg Combination therapy: telmisartan + amlodipine |
| Outcomes | Primary: onset of atrial fibrillation |
| Notes | We asked if the study met our inclusion criteria but received no response |
VALIANT 2003.
| Methods | Multicentre, randomised, double‐blind, event‐driven, clinical trial Median follow‐up: 24.7 months |
| Participants | Inclusion criteria: individuals aged ≥ 18 years who had had acute myocardial infarction (0.5 to 10 days previously) that was complicated by clinical or radiological signs of heart failure or evidence of left ventricular systolic dysfunction Countries: Argentina, Australia, Austria, Belgium, Brazil, Canada, the Czech Republic, Denmark, France, Germany, Hungary, Ireland, Italy, the Netherlands, New Zealand, Norway, Poland, Russia, Slovakia, South Africa, Spain, Sweden, the UK, the USA |
| Interventions | Monotherapy 1: valsartan 20 mg twice daily Monotherapy 2: captopril 6.25 mg 3 times daily Combination therapy: valsartan 20 mg twice daily + captopril 6.25 mg 3 times daily Doses were gradually increased with the goal of reaching valsartan 160 mg, captopril 50 mg, or valsartan 80 mg + captopril 50 mg at 3 months. Investigators increased or decreased the doses of the study drugs at their discretion according to the participant's clinical status |
| Outcomes | Primary: all‐cause mortality Secondary: cardiovascular death, acute coronary syndromes, cardiovascular morbidity, revascularisation procedures, cardiovascular procedures, hospitalisations, adverse events |
| Notes | Participants were candidates to also receive beta‐blockers Guidelines discourage the studied combination We requested data for outcomes of interest for the subgroup of people with hypertension without previous treatment and without additional antihypertensive drugs but received no response |
HbA1c: glycated haemoglobin; PON‐1: Paraoxonase‐1.
Differences between protocol and review
We have improved the wording of the title of the review from "Monotherapy versus combination therapy used as first‐line therapy for primary hypertension" to "First‐line combination therapy versus first‐line monotherapy for primary hypertension".
We have corrected the unit of analysis from "individual trials" to "individual participants".
We did not perform the subgroup analysis with people aged 75 years or over, due to insufficient data.
Contributions of authors
JG, the lead author of the review, entered the text of the review into Review Manager 5, appraised inclusion criteria and quality, and extracted and analysed data.
LCS co‐ordinated the review, conducted the external correspondence, appraised inclusion criteria and quality, and extracted and analysed data.
AA appraised inclusion criteria and quality of studies, and drafted the final review.
IG appraised inclusion criteria and quality of studies, and interpreted the analysis from a methodological and policy perspective.
MJA appraised inclusion criteria and quality of studies, and interpreted the analysis from a clinical perspective.
JE appraised inclusion criteria and quality of studies, and drafted the final review.
All authors participated in the writing of the Discussion and Authors' conclusions.
Sources of support
Internal sources
-
Navarre Health Service and Health Department of the Government of Navarre, Spain
Working time of authors (employees of the Government of Navarre). Facilities. Library services.
External sources
-
University of British Columbia, Vancouver, Canada
Bibliographic searches. Methodological support.
-
European Social Fund Operational Programme 2007 to 2013, Other
50% of the full research project, as salary from September 2012 to December 2015 for the Pharmacotherapy Research Coordinator in the Navarre Health Service (LCS).
Declarations of interest
Javier Garjón: None known.
Luis Carlos Saiz: None known.
Ana Azparren: None known.
Idoia Gaminde: None known.
M José Ariz: None known.
Juan Erviti: None known.
Edited (no change to conclusions)
References
References to studies included in this review
BENEDICT‐A 2004 {published and unpublished data}
- BENEDICT Group. The BErgamo NEphrologic DIabetes Complications Trial (BENEDICT): design and baseline characteristics. Controlled Clinical Trials 2003;24(4):442-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Fassi A, Ilieva A, Iliev IP, Chiurchiu C, Rubis N, et al. Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. Journal of Hypertension 2011;29:207-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, et al. Preventing microalbuminuria in type 2 diabetes. New England Journal of Medicine 2004;351(19):1941-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
PREMIER 2003 {published and unpublished data}
- Mogensen CE, Viberti G, Halimi S, Ritz E, Ruilope L, Jermendy G, et al. Effect of low-dose perindopril/indapamide on albuminuria in diabetes: preterax in albuminuria regression: PREMIER. Hypertension 2003;41(5):1063-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
PREVER‐treatment 2016 {published and unpublished data}
- Fuchs FD, Fuchs SC, Moreira LB, Gus M, Nobrega AC, Poli-de-Figueiredo CE, et al. A comparison between diuretics and angiotensin-receptor blocker agents in patients with stage I hypertension (PREVER-treatment trial): study protocol for a randomized double-blind controlled trial. Trials 2011;12:53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs FD, Scala LC, Vilela-Martin JF, Mello RB, Mosele F, Whelton PK, et al. Effectiveness of chlorthalidone/amiloride versus losartan in patients with stage I hypertension: results from the PREVER-treatment randomized trial. Journal of Hypertension 2016;34(4):798-806. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fuchs FD, Scala LC, Vilela-Martin JF, Whelton PK, Poli-de-Figueiredo CE, Pereira ES, et al. Effectiveness of chlorthalidone/amiloride versus losartan in patients with stage I hypertension and diabetes mellitus: results from the PREVER-treatment randomized controlled trial. Acta Diabetologica 2021;58(2):215-20. [DOI: 10.1007/s00592-020-01611-8] [DOI] [PubMed] [Google Scholar]
REASON 2001 {published and unpublished data}
- Asmar RG, London GM, O'Rourke ME, Mallion JM, Romero R, Rahn KH, et al. Amelioration of arterial properties with a perindopril-indapamide very-low-dose combination. Journal of Hypertension. Supplement 2001;19(4):S15-20. [PMID: ] [PubMed] [Google Scholar]
- Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension 2001;38(4):922-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- London GM, Asmar RG, O'Rourke MF, Safar ME. Mechanism(s) of selective systolic blood pressure reduction after a low-dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenolol. Journal of the American College of Cardiology 2004;43(1):92-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mallion JM, Chamontin B, Asmar R, De Leeuw PW, O'Brien E, Duprez D, et al. Twenty-four-hour ambulatory blood pressure monitoring efficacy of perindopril/indapamide first-line combination in hypertensive patients: the REASON study. American Journal of Hypertension 2004;17(3):245-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACCELERATE 2011 {published data only}
- Brown MJ, McInnes GT, Papst CC, Zhang J, MacDonald TM. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial. Lancet 2011;377(9762):312-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
BENEDICT‐B 2011 {published and unpublished data}
- Ruggenenti P, Fassi A, Ilieva A, Iliev IP, Chiurchiu C, Rubis N, et al. Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. Journal of Hypertension 2011;29(2):207-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
DEMAND 2011 {published and unpublished data}
- Ruggenenti P, Lauria G, Iliev IP, Fassi A, Ilieva AP, Rota S, et al. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the Delapril and Manidipine for Nephroprotection in Diabetes (DEMAND) randomized clinical trial. Hypertension 2011;58(5):776-83. [DOI] [PubMed] [Google Scholar]
MRC‐O 1992 {published data only}
- MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ (Clinical Research Ed.) 1992;304(6824):405-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ONTARGET 2008 {published data only}
- Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. New England Journal of Medicine 2008;358(15):1547-59. [DOI] [PubMed] [Google Scholar]
PATHWAY‐1 2017 {published data only}
- MacDonald TM, Williams B, Webb DJ, Morant S, Caulfield M, Cruickshank JK, et al. Combination therapy is superior to sequential monotherapy for the initial treatment of hypertension: a double-blind randomized controlled trial. Journal of the American Heart Association 2017;6(11):e006986. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PICXEL 2005 {published and unpublished data}
- Dahlof B, Gosse P, Gueret P, Dubourg O, Simone G, Schmieder R, et al. Perindopril/indapamide combination more effective than enalapril in reducing blood pressure and left ventricular mass: the PICXEL study. Journal of Hypertension 2005;23(11):2063-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
PREVER‐prevention 2016 {published data only}
- Fuchs SC, Poli-de-Figueiredo CE, Figueiredo Neto JA, Scala LC, Whelton PK, Mosele F, et al. Effectiveness of chlorthalidone/amiloride versus losartan in patients with stage I hypertension: results from the PREVER-treatment randomized trial. Journal of the American Heart Association 2016;5:e004248. [DOI: 10.1161/JAHA.116.004248] [DOI] [Google Scholar]
Zhang 2010 {published data only}
- Zhang JL, Qin YW, Zheng X, Qiu JL, Zhao XX, Zou DJ. Combination therapy with angiotensin-converting enzyme inhibitors and indapamide impairs glucose tolerance in Chinese hypertensive patients. Blood Pressure 2010;19(2):110-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Derosa 2013 {published data only}
- Derosa G, Cicero AF, Carbone A, Querci F, Fogari E, D'Angelo A, et al. Olmesartan/amlodipine combination versus olmesartan or amlodipine monotherapies on blood pressure and insulin resistance in a sample of hypertensive patients. Clinical and Experimental Hypertension 2013;35(5):301-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Derosa 2014 {published data only}
- Derosa G, Bonaventura A, Romano D, Bianchi L, Fogari E, D'Angelo A, et al. Enalapril/lercanidipine combination on markers of cardiovascular risk: a randomized study. Journal of the American Society of Hypertension 2014;8(6):422-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Derosa 2016 {published data only}
- Derosa G, Mugellini A, Pesce RM, D'Angelo A, Maffioli P. Olmesartan combined with amlodipine on oxidative stress parameters in type 2 diabetics, compared with single therapies: a randomized, controlled, clinical trial. Medicine 2016;95(13):e3084. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
INSIGHT 2000 {published data only}
- Brown MJ, Palmer CR, Castaigne A, Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000;356(9227):366-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Maeda 2009 {published data only}
- Maeda S, Nishizaki M, Yamawake N, Shimada H, Asano M, Ihara K, et al. Telmisartan prevents the development of atrial fibrillation in hypertensive patients. Heart Rhythm 2009;6(Suppl 5):S334. [Google Scholar]
VALIANT 2003 {published data only}
- Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. New England Journal of Medicine 2003;349(20):1893-906. [DOI] [PubMed] [Google Scholar]
Additional references
Access 2010 [Computer program]
- Microsoft Access. Microsoft Corporation, 2010. Available at: office.microsoft.com/access.
ACCOMPLISH 2008
- Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. New England Journal of Medicine 2008;359(23):2417-28. [DOI: 10.1056/NEJMoa0806182] [DOI] [PubMed] [Google Scholar]
ALTITUDE 2012
- Parving HH, Brenner BM, McMurray JJV, Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. New England Journal of Medicine 2012;367(23):2204-13. [DOI: 10.1056/NEJMoa1208799] [DOI] [PubMed] [Google Scholar]
Bakris 2014
- Bakris G, Sarafidis P, Agarwal R, Ruilope L. Review of blood pressure control rates and outcomes. Journal of the American Society of Hypertension 2014;8(2):127-41. [DOI: 10.1016/j.jash.2013.07.009] [DOI] [PubMed] [Google Scholar]
Chen 2018
- Chen YJ, Li LJ, Tang WL, Song JY, Qiu R, Li Q, et al. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD008170. [DOI: 10.1002/14651858.CD008170.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, 2019. Available at covidence.org.
EMA/294911/2014
- European Medicines Agency. Combined use of medicines affecting the renin-angiotensin system (RAS) to be restricted - CHMP endorses PRAC recommendation, 2014. www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/05/WC500167421.pdf.
EROS [Computer program]
- EROS Early Review Organizing Software. Version 2.0. Buenos Aires: IECS (Institute of Clinical Effectiveness and Health Policy), 2009. Available at www.eros-systematic-review.org/.
ESH/ESC 2013
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2013;34(28):2159-219. [DOI: 10.1093/eurheartj/eht151] [DOI] [PubMed] [Google Scholar]
ESH/ESC 2018
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. European Heart Journal 2018;39(33):3021-104. [DOI: 10.1093/eurheartj/ehy339] [DOI] [PubMed] [Google Scholar]
Excel 2010 [Computer program]
- Microsoft Excel. Microsoft Corporation, 2010. Available at: office.microsoft.com/excel.
FDA 2014
- US Food and Drug Administration. ACE inhibitors: dual blockade of the RAS. wayback.archive-it.org/7993/20170112173259/http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm418829.htm (accessed 5 August 2015).
Fu 2017
- Fu S, Wen X, Han F, Long Y, Xu G. Aliskiren therapy in hypertension and cardiovascular disease: a systematic review and a meta-analysis. Oncotarget 2017;8(51):89364-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gradman 2010
- Gradman AH, Basile JN, Carter BL, Bakris GL, Materson BJ, Black HR, et al. Combination therapy in hypertension. Journal of the American Society of Hypertension 2010;4(2):90-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
He 2017
- He T, Liu X, Li Y, Liu XY, Wu QY, Liu ML, et al. High-dose calcium channel blocker (CCB) monotherapy vs combination therapy of standard-dose CCBs and angiotensin receptor blockers for hypertension: a meta-analysis. Journal of Human Hypertension 2017;31(2):79-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Heran 2012
- Heran BS, Chen JM, Wang JJ, Wright JM. Blood pressure lowering efficacy of potassium-sparing diuretics (that block the epithelial sodium channel) for primary hypertension. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD008167. [DOI: 10.1002/14651858.CD008167.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from.
Hilleman 1999
- Hilleman DE, Ryschon KL, Mohiuddin SM, Wurdeman RL. Fixed-dose combination vs monotherapy in hypertension: a meta-analysis evaluation. Journal of Human Hypertension 1999;13(7):477-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hopkins 2010
- Hopkins KA, Bakris GL. Fixed-dose combination and chronic kidney disease progression: which is the best? Current Opinion in Nephrology and Hypertension 2010;19(5):450-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hypertension Canada 2020
- Rabi DM, McBrien KA, Sapir-Pichhadze R, Nakhla M, Ahmed SB, Dumanski SM, et al. Hypertension Canada's 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Canadian Journal of Cardiology 2020;36(5):596-624. [DOI: 10.1016/j.cjca.2020.02.086] [DOI] [PubMed] [Google Scholar]
ICH 1995
- International Conference on Harmonisation. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. ICH Harmonised Tripartite Guideline CPMP/ICH/377/95. London: European Medicines Agency, 1995. [Google Scholar]
JNC 8 2014
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507-20. [DOI: 10.1001/jama.2013.284427] [PMID: ] [DOI] [PubMed] [Google Scholar]
Law 2003a
- Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ (Clinical Research Ed.) 2003;326(7404):1427. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2003b
- Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technology Assessment (Winchester, England) 2003;7(31):1-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Law 2009
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ (Clinical Research Ed.) 2009;338:b1665. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2013
- Liu Y, Chen K, Kou X, Han Y, Zhou L, Zeng C. Aliskiren and amlodipine in the management of essential hypertension: meta-analysis of randomized controlled trials. PLoS ONE 2013;8(7):e70111. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lv 2010
- Lv Y, Zou Z, Chen GM, Jia HX, Zhong J, Fang WW. Amlodipine and angiotensin-converting enzyme inhibitor combination versus amlodipine monotherapy in hypertension: a meta-analysis of randomized controlled trials. Blood Pressure Monitoring 2010;15(4):195-204. [PMID: ] [DOI] [PubMed] [Google Scholar]
Makani 2013
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ (Clinical Research Ed.) 2013;346:f360. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management (NG136). London: National Institute for Health and Care Excellence, August 2019. [Google Scholar]
Norris 2010
- Norris S, Weinstein J, Peterson K, Thakurta S. Drug Class Review: Direct Renin Inhibitors, Angiotensin Converting Enzyme Inhibitors, and Angiotensin II Receptor Blockers: Final Report. Portland (OR): Oregon Health & Science University, January 2010. [PMID: ] [PubMed] [Google Scholar]
PARTAGE 2015
- Benetos A, Labat C, Rossignol P, Fay R, Rolland Y, Valbusa F, et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: the PARTAGE study. JAMA Internal Medicine 2015;175(6):989-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Povoa 2014
- Povoa R, Barroso WS, Brandao AA, Jardim PC, Barroso O, Passarelli O Jr, et al. I Brazilian position paper on antihypertensive drug combination. Arquivos Brasileiros de Cardiologia 2014;102(3):203-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rea 2018
- Rea F, Corrao G, Merlino L, Mancia G. Early cardiovascular protection by initial two-drug fixed-dose combination treatment vs. monotherapy in hypertension. European Heart Journal 2018;39(40):3654-61. [DOI: 10.1093/eurheartj/ehy420] [DOI] [PubMed] [Google Scholar]
Reboussin 2018
- Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2018;71(19):2176-98. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Ruzicka 2001
- Ruzicka M, Leenen FH. Monotherapy versus combination therapy as first line treatment of uncomplicated arterial hypertension. Drugs 2001;61(7):943-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scott 2015
- Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Internal Medicine 2015;175(5):827-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sood 2010
- Sood N, Reinhart KM, Baker WL. Combination therapy for the management of hypertension: a review of the evidence. American Journal of Health-System Pharmacy 2010;67(11):885-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wald 2009
- Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. American Journal of Medicine 2009;122(3):290-300. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weir 2017
- Weir S, Juhasz A, Puelles J, Tierney TS. Relationship between initial therapy and blood pressure control for high-risk hypertension patients in the UK: a retrospective cohort study from the THIN general practice database. BMJ Open 2017;7:e015527. [DOI: 10.1136/bmjopen-2016-015527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whelton 2018
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2018;71(19):e127-e248. [DOI: 10.1016/j.jacc.2017] [DOI] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta-blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD002003. [DOI: 10.1002/14651858.CD002003.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2018
- Wright JM, Musini VM, Gill R. First-line drugs for hypertension. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD001841. [DOI: 10.1002/14651858.CD001841.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2021
- Zhu J, Chen N, Zhou M, Guo J, Zhu C, Zhou J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD003654. [DOI: 10.1002/14651858.CD003654.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Garjón 2017
- Garjón J, Saiz LC, Azparren A, Elizondo JJ, Gaminde I, Ariz MJ, et al. First-line combination therapy versus first-line monotherapy for primary hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD010316. [DOI: 10.1002/14651858.CD010316.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


